1. Background
Coronavirus disease 2019 (COVID-19), caused by the latest known virus of the coronavirus family, emerged as a global health threat following its identification in Wuhan, China, in December 2019 (1). On January 12, 2020, the World Health Organization (WHO) provisionally named this virus novel coronavirus-2019. Subsequently, on January 30, 2020, the WHO declared the outbreak a Public Health Emergency of International Concern. On February 11, 2020, the disease was officially named COVID-19 (2). The rapid global spread of COVID-19, including its significant impact in Iran, has posed substantial risks to both public mental and physical health.
Pregnant women and their fetuses represent a high-risk population in the context of infectious diseases such as COVID-19 (3). Additionally, infants require particular attention due to their potential for asymptomatic infection or the presentation of mild to severe symptoms (4). The clinical manifestations of COVID-19 in infants can be atypical and develop gradually (5, 6). Elevated body temperature, often observed, is complicated by temperature instability, especially in premature infants (3). Typical adult symptoms, such as tachypnea, apnea, and cough, may not be reliable indicators of COVID-19 in infants. Instead, non-specific symptoms like feeding refusal, lethargy, diarrhea, and vomiting are frequently observed (3). Diagnosis in this age group is primarily established via RT-PCR testing of nasal or nasopharyngeal samples (7).
Coronavirus disease 2019 may alter immune responses in the mother-fetus relationship, impacting maternal and neonatal well-being. Managing infected pregnant women is complex and challenging due to the potential negative effects on the mother, fetus, and infant (8, 9). Reports indicate risks such as premature delivery, premature rupture of membranes (PROM), fetal tachycardia, and fetal distress, particularly when infection occurs during the third trimester. However, current evidence does not suggest an increased risk of miscarriage due to COVID-19 (10).
Concerns about congenital infection have led some clinicians to consider pregnancy termination, though comprehensive data on COVID-19’s effects on pregnancy processes and outcomes remain limited. Variability in maternal outcomes may be associated with the stage of pregnancy, maternal age, medication use, and differences in immune responses. Adverse pregnancy outcomes reported include maternal death, stillbirth, spontaneous miscarriage, premature delivery, low birth weight, PROM, fetal distress, and severe fever during the first trimester, which may increase the risk of congenital defects (11).
A systematic review by Irani et al. underscores the limited findings and insufficient data regarding adverse pregnancy and neonatal outcomes among COVID-19-infected pregnant women (12). Given the vulnerability of pregnant women and neonates during the COVID-19 pandemic, it is crucial to prioritize this population in prevention and management strategies.
2. Objectives
The present study aims to elucidate maternal and neonatal outcomes among COVID-19-infected pregnant women.
3. Methods
This descriptive, analytical, cross-sectional case-control study aimed to determine maternal and neonatal outcomes among pregnant women infected with COVID-19 during the third trimester. The research was conducted at hospitals affiliated with Shiraz University of Medical Sciences, the primary referral center in southern Iran, from March 2021 to March 2022. All women who met the inclusion criteria were enrolled in the study.
The study included pregnant women diagnosed with COVID-19 during their third trimester and their neonates. A control group was established, comprising women and their neonates matched for maternal age, parity, and third trimester of pregnancy, born at the same hospital but not infected with COVID-19.
Infants born to mothers diagnosed with COVID-19 during the third trimester of pregnancy were enrolled in the study. Exclusion criteria included mothers with chronic or inflammatory diseases before pregnancy, such as diabetes, hypertension, cardiovascular, liver, or kidney diseases, as well as incomplete medical records.
Data were collected using a pre-designed checklist and a review of medical records. The checklist included the following.
3.1. Maternal Demographics, Clinical and Pregnancy Data
Maternal age, gravidity, type of delivery, clinical manifestations (fever, sore throat, cough, diarrhea, myalgia, fatigue), antiviral therapy, duration between onset of symptoms and delivery, history of contact with an infected person, and other relevant medical conditions (gestational diabetes, preeclampsia, vaginal bleeding, PROM), fetal distress, abnormal umbilical cord, placenta previa, oligohydramnios, polyhydramnios, and preterm delivery.
3.2. Neonatal Outcomes
Birth weight, Apgar score at 5 minutes, NICU admission, respiratory conditions [respiratory distress syndrome (RDS], transient tachypnea of the newborn (TTN), neonatal asphyxia, and other health conditions (meconium staining, chorioamnionitis, pneumonia, disseminated intravascular coagulation, multi-organ failure, septic shock, gastrointestinal bleeding, etc.). Additional data included findings of chest X-rays (CXR), neonatal signs and symptoms, and laboratory data such as vomiting, rash, thrombocytopenia, abnormal liver function tests (LFT), complete blood count (CBC), C-reactive protein (CRP), and COVID-19 PCR results.
Data analysis was conducted using SPSS version 25. Descriptive statistics were used to summarize the data, with means and standard deviations reported for continuous variables and counts and percentages for categorical variables. The normality of data was assessed using the Shapiro-Wilk test. For categorical comparisons, the chi-square test was applied. For continuous variables, independent t-tests were used for normally distributed data, and the Mann-Whitney U test was applied for non-normally distributed data. Comparative analyses between the COVID-19 and control groups employed the chi-square test for categorical variables and appropriate tests based on normality for continuous variables. A P-value < 0.05 was considered statistically significant.
4. Results
This study compared pregnancy outcomes between women with and without COVID-19, analyzing data from 346 women (173 in each group).
The median ages of pregnant women with and without COVID-19 were 31.5 and 30.5 years, respectively, with no significant difference in age between the groups (P = 0.131). Both groups predominantly consisted of Iranian women, with no significant ethnic differences observed (P = 0.72). Nulliparous women formed the largest subgroup in both cohorts, accounting for 38.4% of COVID-19 cases and 36.9% of controls, with parity distribution being similar across the groups (P = 0.5). Detailed comparisons of patient characteristics are provided in Table 1.
| Characteristics | Pregnant Women with a Clinical Diagnosis of COVID‐19 (n = 173) | Pregnant Women Without COVID‐19 (n = 173) | P-Value b |
|---|---|---|---|
| Age (y) | 31.5 ± 5.9 | 30.5 ± 6.6 | 0.13 c |
| Gestational age | 0.01 d | ||
| Term | 102 (59) | 129 (74.5) | |
| Preterm | 71 (41) | 44 (25.5) | |
| Mode of delivery | 0.21 d | ||
| Vaginal delivery | 32 (18.5) | 48 (27.8) | |
| Cesarean section | 141 (81.5) | 125 (72.2) | |
| Clinical manifestations | - | ||
| Fever | 63 (36.4) | - | |
| Cough | 128 (73.9) | - | |
| Sore throat | 50 (28.9) | - | |
| Diarrhea | 3 (1.7) | - | |
| Myalgia | 83 (47.9) | - | |
| Fatigue | 34 (19.6) | - | |
| Antiviral therapy | 65 (37.6) | - | - |
| The interval between the onset of COVID-19 symptoms and delivery (day) | 6.3 | - | - |
| Contact with an infected person | 97 (56) | - | - |
a Values are expressed as No. (%) unless otherwise indicated.
b The P-value less than 0.05 is considered as statistically significant.
c Assessed by t-test.
d Assessed by chi-square test.
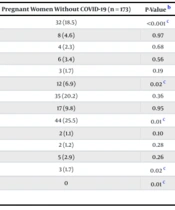
Vaginal bleeding was significantly more common among COVID-19 patients (14.5% vs. 6.9%, P = 0.021). Fetal distress was markedly higher in the COVID-19 group (43.4% vs. 18.5%, P < 0.001). ICU admissions were significantly more frequent among women with COVID-19 (3.5% vs. 0%, P = 0.014). Stillbirth occurred more often in COVID-19 cases (6.4% vs. 1.7%, P = 0.027). These maternal outcomes are summarized in Table 2.
| Outcome | Pregnant Women with a Clinical Diagnosis of COVID‐19 (n = 173) | Pregnant Women Without COVID‐19 (n = 173) | P-Value b |
|---|---|---|---|
| Fetal distress | 75 (43.4) | 32 (18.5) | < 0.001 c |
| Abnormal umbilical cord | 8 (4.6) | 8 (4.6) | 0.97 |
| Placenta previa | 2 (1.2) | 4 (2.3) | 0.68 |
| Oligohydramnios | 8 (4.6) | 6 (3.4) | 0.56 |
| Polyhydramnios | 7 (4.0) | 3 (1.7) | 0.19 |
| Vaginal bleeding | 25 (14.5) | 12 (6.9) | 0.02 c |
| Gestational diabetes | 28 (16.2) | 35 (20.2) | 0.36 |
| PROM | 17 (9.8) | 17 (9.8) | 0.95 |
| Preterm delivery | 71 (41) | 44 (25.5) | 0.01 c |
| Meconium staining | 7 (4) | 2 (1.1) | 0.10 |
| Chorioamnionitis | 5 (2.9) | 2 (1.2) | 0.28 |
| Preeclampsia | 9 (5.2) | 5 (2.9) | 0.26 |
| Stillbirth | 11 (6.4) | 3 (1.7) | 0.02 c |
| ICU admission | 6 (3.5) | 0 | 0.01 c |
Abbreviation: PROM, premature rupture of membranes.
a Values are expressed as No. (%).
b Assessed by chi-square test.
c The P-value less than 0.05 is considered as statistically significant.
Low birth weight was significantly more prevalent in neonates born to mothers with COVID-19 (39.3% vs. 19%, P = 0.038). Transfer to the NICU was also significantly higher among neonates from the COVID-19 group (45.1% vs. 20.2%, P < 0.001). However, there was no significant difference in the incidence of low Apgar scores at 5 minutes between the two groups (P = 0.92). These neonatal outcomes are presented in Table 3.
| Variables | Babies Born to Mother with COVID-19 | Babies Born to Mothers Without COVID-19 | P-Value b |
|---|---|---|---|
| Low birth weight | 68 (39.3) | 33 (19) | 0.03 c |
| Apgar at 5 min < 7 | 33 (19) | 28 (16.1) | 0.92 |
| Transferred to NICU | 78 (45.1) | 35 (20.2) | < 0.001 c |
| RDS | 35 (20.2) | 27 (15.6) | 0.23 |
| TTN | 15 (8.7) | 7 (4) | 0.71 |
| Neonatal Asphyxia | 2 (1.2) | 0 (0) | 0.24 |
| Ventilation | 29 (16.8) | 20 (11.5) | 0.14 |
| Pneumothorax | 0 (0) | 0 (0) | - |
| Pneumonia | 9 (5.2) | 4 (2.3) | 0.14 |
| Tachycardia | 3 (1.7) | 0 (0) | 0.12 |
| Respiratory distress | 50 (28.9) | 34 (19.6) | 0.10 |
| Disseminated intravascular coagulation | 5 (2.9) | 1 (0.57) | 0.11 |
| Gastrointestinal bleeding | 0 (0) | 0 (0) | - |
| Multi-organ failure | 0 (0) | 1 (0.57) | > 0.99 |
| Septic shock | 3 (1.7) | 0 (0) | 0.12 |
| CXR | 0.15 | ||
| PNEUMONIA | 9 (5.2) | 4 (2.3) | |
| TTN | 15 (8.6) | 7 (4) | |
| RDS | 34 (19.8) | 24 (13.8) | |
| Not done | 115 (66.4) | 138 (79.9) | |
| Vomiting | 7 (4) | 1 (0.57) | 0.36 |
| Rash | 4 (2.3) | 3 (1.7) | 0.72 |
| Thrombocytopenia | 5 (2.9) | 4 (2.3) | 0.50 |
| Abnormal LFT | 0 (0) | 0 (0) | - |
| White blood cell count | 11604.43 | 11990.7 | 0.25 |
| Lymphocyte | 2080 | 2093.72 | 0.95 |
| CRP | > 0.99 | ||
| Positive | 5 (2.8) | 3 (1.7) | |
| Negative | 75 (43.3) | 32 (18.5) | |
| Not measured | 93 (53.9) | 138 (79.8) | |
| Positive COVID-19 PCR | 3 (1.7) | 0 (0) | - |
| Not checked | 0 (0) | 173 (100) | - |
Abbreviations: CXR, chest X-rays; TTN, transient tachypnea of the newborn; RDS, respiratory distress syndrome.
a Values are expressed as No. (%).
b Assessed by chi-square test.
c A P-value less than 0.05 is considered as statistically significant.
5. Discussion
In this study, significant differences were observed in pregnancy outcomes between women with and without COVID-19. Women with COVID-19 had a higher incidence of preterm delivery (41.0% vs. 25.5%, P = 0.01), fetal distress (43.4% vs. 18.5%, P < 0.001), and stillbirth (6.4% vs. 1.7%, P = 0.027). They also experienced higher rates of vaginal bleeding (14.5% vs. 6.9%, P = 0.021) and ICU admission (3.5% vs. 0%, P = 0.014). Regarding neonatal outcomes, babies born to mothers with COVID-19 had a higher incidence of low birth weight (39.3% vs. 19%, P = 0.038) and more frequent NICU transfers (45.1% vs. 20.2%, P < 0.001). These findings underscore the increased risks of adverse maternal and neonatal outcomes associated with COVID-19 infection during pregnancy. The study emphasizes the importance of close monitoring and comprehensive management of pregnant women with COVID-19 to mitigate these risks.
In the current study, the most common c(13-16)linical symptoms in COVID-19-positive mothers were cough (73.9%), myalgia (47.9%), and fever (36.4%). These findings align with previous research identifying fever (87.5%) and cough (53.8%) as predominant symptoms of COVID-19, while less frequent symptoms included diarrhea and anorexia (13-16). Similarly, Chen et al. reported fever and cough as the primary symptoms among nine pregnant women diagnosed with COVID-19, consistent with our findings (17). These commonalities confirm that the typical symptom profile of COVID-19 in pregnant women includes respiratory and systemic symptoms, with gastrointestinal symptoms being less common.
In this study, 81.5% of women with COVID-19 underwent cesarean section. This high rate was influenced by medical complications such as preeclampsia, hypertension, gestational diabetes, and shortness of breath associated with the infection. Additionally, personal preferences and concerns about potential virus transmission from mother to child during vaginal delivery contributed to this trend. However, evidence from other studies suggests that vaginal delivery does not increase the risk of COVID-19 transmission to medical staff or the infant. These studies recommend that the mode of delivery should be guided by obstetric indications rather than concerns over viral transmission, as there is no significant increase in risk associated with vaginal delivery (18-21).
The current study provides critical insights into maternal and neonatal outcomes associated with COVID-19 during pregnancy, revealing significant differences in several key areas. Notable findings include increased rates of vaginal bleeding, fetal distress, preterm birth, intrauterine death, ICU admissions, low birth weight, and NICU admissions among women with COVID-19 and their infants. These outcomes align with previous studies, which have also identified heightened risks for complications such as fetal distress, PROM, and preterm birth among pregnant women with COVID-19 (22-26). For instance, a multicenter study reported that 12% of infants born to mothers with COVID-19 were admitted to the NICU, 10% were preterm, and 3% required mechanical ventilation, underscoring elevated risks for NICU admissions and prematurity (23). Another study in Iran by Jahansuz et al. similarly noted a higher rate of preterm births among mothers with COVID-19 (27). Additionally, the study by Gholami et al. highlighted clinically significant risks such as preeclampsia, gestational diabetes, cesarean section, preterm birth, and NICU admission, alongside a notable decrease in mean gestational age compared to the previous year (28). Vizheh et al. also reported higher rates of preterm births, increased cesarean sections, and more frequent NICU admissions among neonates born to COVID-19-positive mothers (29).
Despite these findings, our study did not establish a significant relationship between maternal COVID-19 status and certain neonatal outcomes such as TTN and pneumonia. However, these conditions were observed more than twice as frequently in infants born to COVID-19-positive mothers (23, 30-33). Other studies have similarly noted trends where abnormalities, such as abnormal lung X-rays, thrombocytopenia, and positive CRP, were slightly more common in infants of mothers with COVID-19, though these differences did not reach statistical significance (22, 34, 35).
Additionally, while the high rate of cesarean sections in our study reflects both medical and non-medical reasons, such as preeclampsia and hypertension, previous research suggests that vaginal delivery does not pose a significant risk of virus transmission from mother to child (3, 12, 17). This finding underscores that the choice of delivery method should prioritize obstetric indications rather than concerns about viral transmission. A study conducted in Kurdistan Province found that cesarean section rates increased from 34% before the COVID-19 pandemic to 37% afterward. Monthly data from March 2018 to January 2023 revealed fluctuations in cesarean rates before and after the pandemic onset, with a general upward trend following COVID-19’s emergence. Specifically, cesarean delivery rates among primigravid women surged at the pandemic’s onset and maintained a gradual increase overall. This trend highlights the pandemic’s impact on delivery methods across various groups of pregnant women (36).
This study has several strengths, including a larger sample size and a robust case-control design, enabling a comprehensive analysis of maternal and neonatal outcomes in COVID-19-positive pregnant women. This methodological approach facilitated the clear identification of specific risks associated with COVID-19 during pregnancy, particularly in the third trimester. The inclusion of a matched control group of COVID-19-negative pregnant women further strengthens the reliability and validity of the findings.
However, the study also has important limitations. A key limitation is the potential for selection bias, as the study only included pregnant women who were in their third trimester at the time of COVID-19 diagnosis. This focus may restrict the generalizability of the findings to earlier stages of pregnancy. Additionally, the retrospective nature of the study prevented the testing of samples from the placenta, amniotic fluid, cord blood, and vaginal mucus, resulting in an incomplete assessment of the potential for vertical transmission. This limitation highlights the need for further studies that include pregnant women infected with COVID-19 during the first and second trimesters and allow for comprehensive testing to better understand the full spectrum of prenatal and perinatal risks associated with the virus.
5.1. Conclusions
The findings of this study contribute to the expanding body of evidence indicating that COVID-19 during pregnancy can significantly affect both maternal and neonatal outcomes. The increased risks of complications, including preterm birth, low birth weight, and the need for neonatal intensive care, highlight the importance of prioritizing pregnancy-specific interventions in pandemic management. Given the potential for adverse short-term and long-term outcomes, it is essential to continue investigating the effects of COVID-19 across all stages of pregnancy. This research will better inform clinical practices and help improve health outcomes for both mothers and their infants.