1. Introduction
Hyperphosphatemic familial tumoral calcinosis (HFTC) is a rare autosomal recessive disorder characterized by ectopic calcifications in periarticular soft tissues, particularly around joints such as the hips, elbows, and shoulders. The condition arises due to a relative deficiency or resistance to fibroblast growth factor 23 (FGF23), leading to hyperphosphatemia (1). Clinically, patients present with firm to hard swellings, and laboratory tests typically reveal elevated phosphate levels with normal calcium, vitamin D, and parathyroid hormone levels (2).
Management of HFTC involves dietary phosphate restriction, the use of phosphate binders, and surgical removal of calcified lesions when necessary (2). Genetic testing is crucial for confirming the diagnosis, as mutations in genes such as GALNT3, Klotho (KL), or FGF23 are commonly associated with HFTC. Identifying these genetic mutations helps guide long-term management and genetic counseling (1, 3).
Patients with HFTC exhibit hyperphosphatemia due to increased proximal tubular phosphate reabsorption while maintaining normal calcium and parathyroid hormone levels. Genetic analyses have identified multiple mutations associated with HFTC, including a novel GALNT3 variant related to maternal uniparental disomy of chromosome 2 (3).
Treatment strategies include a combination of dietary phosphate restriction, phosphate-lowering agents, and surgical excision of periarticular masses to achieve remission and improve functional outcomes (4). Diagnosis prioritizes elevated phosphate levels rather than high calcium levels and requires differentiation from conditions such as pseudohypoparathyroidism (5).
Genetic testing is a valuable tool in diagnosing HFTC, particularly for identifying mutations in FGF23, GALNT3, or Klotho, which are crucial for phosphate metabolism (1). In regions lacking genetic testing facilities, diagnosis can be challenging, but genetic analysis remains key to identifying mutations such as GALNT3 variants and planning appropriate treatment (4, 6). This case report underscores the importance of early detection, management, and genetic counseling in effectively addressing HFTC and highlights the need for awareness among medical professionals and the public regarding the complexities of this rare disorder.
2. Case Presentation
This report details the case of a one-year-old child (born July 17, 2022) with normal developmental milestones. The parents were closely related, and the patient was their second child. Following vaccination at six months of age, the child experienced intermittent fevers for two months, followed by progressive swelling in the left gluteal region. However, a definitive association between the patient's clinical presentation and the vaccination process could not be established.
Ultrasound detected a calcified abscess measuring 58 × 28 × 58 mm in the affected area, but no signs of calcification were found in the ultrasonography of both kidneys. Despite negative tuberculosis tests, a biopsy confirmed the presence of a pseudocyst with calcification.
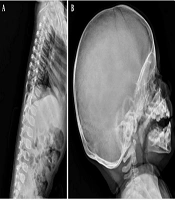
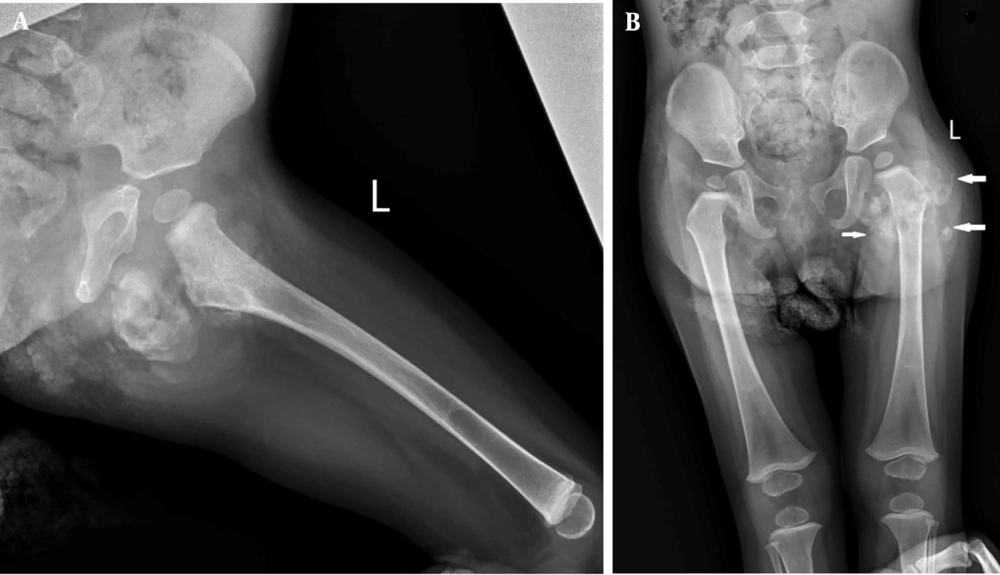
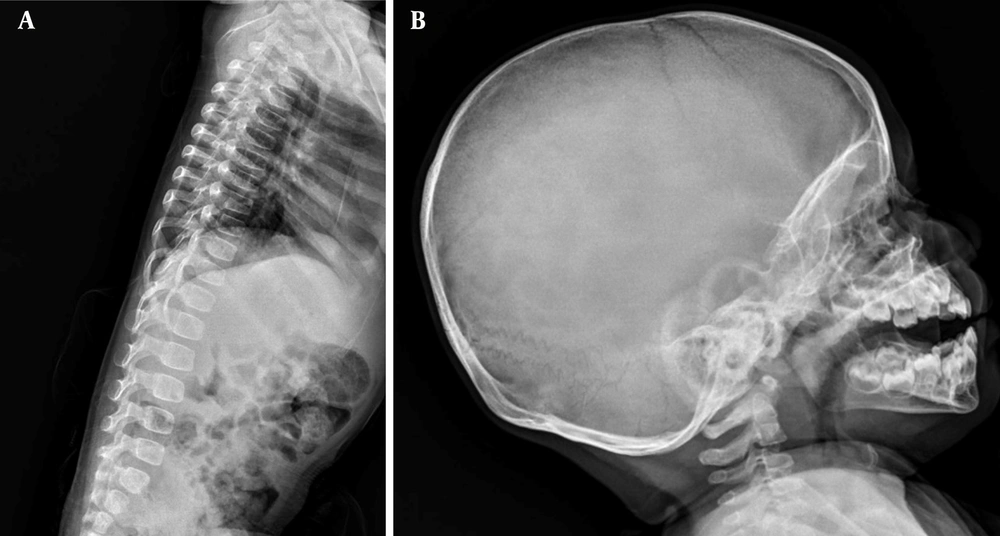
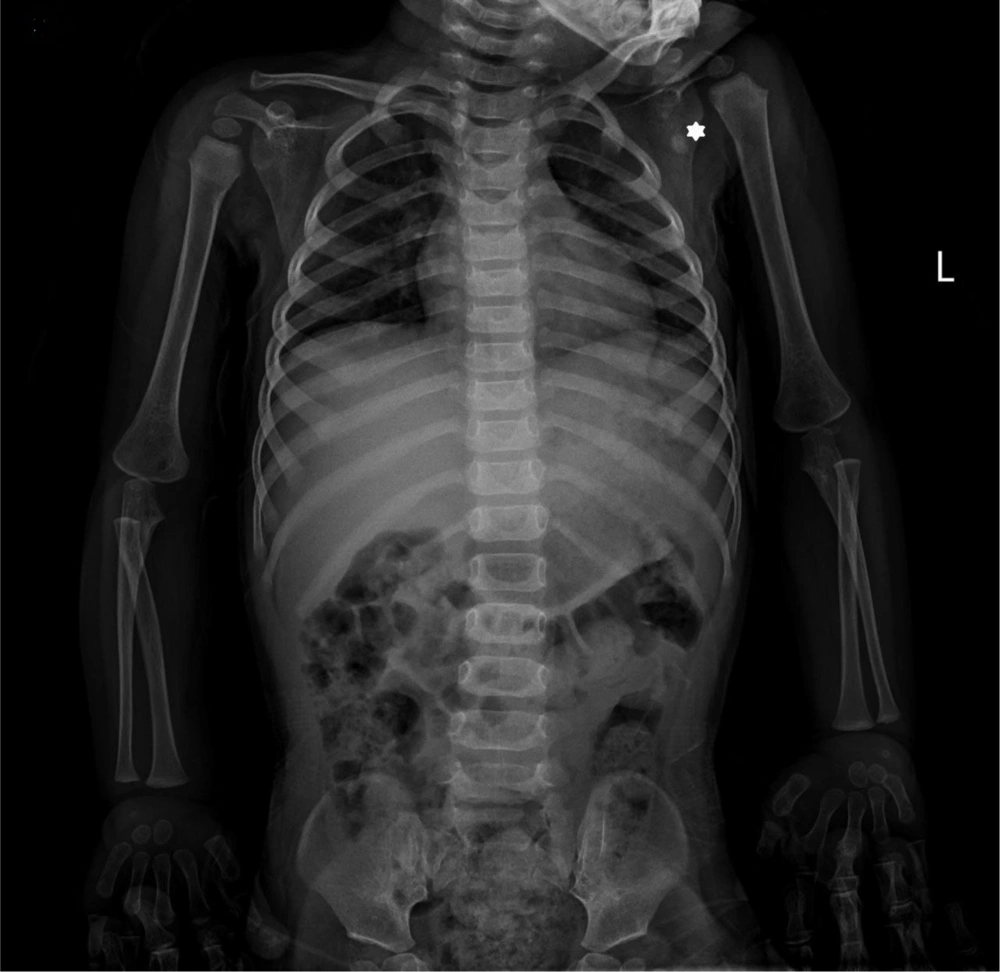
Upon referral to Mofid Children's Hospital (February 26, 2024), most laboratory tests were normal except for elevated calcium, phosphorus, and magnesium levels. A second ultrasound identified a mass measuring 20 × 36 mm. The skeletal survey images revealed a sizable multilobulated soft tissue mass with cloud-like and amorphous calcifications surrounding the left hip joint (indicated by arrows in Figure 1). The adjacent femur bone and hip joint space appeared normal. No pathological findings were present in other parts of the skeleton. Additionally, a calcified left axillary lymph node was observed marked with an asterisk in Figure 3 (Figures 1-3).
2.1. Clinical Manifestation
The one-year-old patient presented with intermittent fevers, a growing mass in the gluteal region (buttock), and limping (Figure 4).
The deposition of calcium in soft tissues, particularly near joints, has been observed and can result in pain, inflammation, and restricted movement, which corresponds to the symptoms experienced by the patient.
2.2. Laboratory Findings
The patient exhibited elevated serum phosphate levels and a normal or slightly elevated 1,25-dihydroxyvitamin D (1,25D) level. Additionally, increased parathyroid hormone (PTH) levels were observed, indicating a potential resistance to fibroblast growth factor 23 (FGF23), which ordinarily functions to suppress PTH secretion and enhance phosphate excretion (Tables 1 - 3). In HFTC, this resistance to FGF23 disrupts its regulatory function, leading to elevated PTH levels and contributing to hyperphosphatemia (Tables 1 - 3).
| Blood levels | October 8 | October 9 | October 13 | October 19 | Unit |
|---|---|---|---|---|---|
| Ca. | 11.2 | 10.5 | 10.9 | 11 | mg/dL |
| Phosphorus | 7.7 | 9.2 | 8.4 | 7.7 | mg/dL |
| Test | Risk | Result | Unit |
|---|---|---|---|
| BUN | 7.4 | (mg/dL) | |
| Creatinine | 0.37 | (mg/dL) | |
| Calcium (Total) | 10.7 | (mg/dL) | |
| Phosphorus (Inorganic) | H | 6.3 | (mg/dL) |
| Na | 137 | (mg/dL) | |
| K | 4.20 | (mg/dL) | |
| ALP | 308 | U/L | |
| Venous blood gas: VBG | |||
| Acid/Base | 7.316 | ||
| PH | 38.5 | ||
| pCO2 | L | -6.4 | mmHg |
| BE | 19.2 | mmol/L | |
| HCO3- | mmol/L | ||
| Hb/Oxygen status | |||
| PO2 | 33.8 | mmHg | |
| tHb | L | 9.7 | g/dL |
| 02 Saturation | L | 57.4 | % |
| WBCs | Result | Risk | Unit |
|---|---|---|---|
| WBC | 12.70 | 103/μL | |
| Neutrophil | 37.80 | % | |
| Lymphocyte | 55.00 | % | |
| Monocyte | 6.80 | % | |
| Eosinophil | 0.20 | % | |
| Basophil | 0.20 | % | |
| Neutrophils # | 4.80 | 103/μL | |
| Lymphocytes # | 6.98 | H | 103/μL |
| Monocyte # | 0.86 | 103/μL | |
| Eosinophil # | 0.03 | 103/μL | |
| Basophil # | 0.03 | 103/μL | |
| RBC | 5.25 | H | 106/μL |
| Hb | 9.7 | L | g/dL |
| Het | 32.2 | L | % |
| MCV | 61.4 | L | fL |
| MCH | 18.2 | L | Pg |
| MCHC | 29.8 | L | g/Dl |
| RDW-CV | 21.1 | H | % |
| RDW-SD | 47.8 | Fl | |
| nRBC% | 0.0 | % | |
| nRBC # | 0.0 | 103/μL | |
| Platelets | |||
| Platelet (*1) | 790 | H | 103/μL |
| MPV | 8.4 | Fl | |
| PDW | 15.9 | % | |
| P-LCR | 19.4 | % |
The analysis of the patient's blood calcium and phosphorus levels from October 8 to October 19 provides important insights into their condition (Table 1). The patient's calcium levels ranged from 10.5 to 11.2 mg/dL, which is at the high end of the normal range (8.5 to 10.5 mg/dL). These levels showed minor fluctuations: They were 11.2 mg/dL on October 8, dropped to 10.5 mg/dL on October 9, rose slightly to 10.9 mg/dL on October 13, and stabilized at 11.0 mg/dL by October 19, indicating mild variability but staying within the upper normal range.
In contrast, the patient's phosphorus levels were consistently elevated, ranging from 7.7 to 9.2 mg/dL, well above the normal range of 2.5 to 4.5 mg/dL. The phosphorus level was 7.7 mg/dL on October 8, increased sharply to 9.2 mg/dL on October 9, decreased to 8.4 mg/dL by October 13, and returned to 7.7 mg/dL on October 19. This persistent elevation in phosphorus levels indicates hyperphosphatemia, which is characterized by abnormally high phosphate levels in the blood.
The test results indicate elevated phosphorus and alkaline phosphatase levels, suggesting potential hyperphosphatemia.
In the table above (Table 4), several results fall outside the normal range. The lymphocyte count is higher than normal, and the red blood cell count is elevated above the typical range. Hemoglobin and hematocrit levels are lower than the standard values. Additionally, the mean cell volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) are below normal, indicating microcytic and hypochromic anemia, while there is increased variation in red blood cell size (anisocytosis). The platelet count is significantly elevated as well, which may suggest a reactive process or underlying inflammation.
| Blood Levels | October 12 | Unit |
|---|---|---|
| CRP | 32 | mg/L |
| ESR | 59 | mm/hr |
| NBT | 100% | Percentage (%) |
| IgG | 75.3 | mg/dL |
| IgA | 45 | mg/dL |
| IgM | 128 | mg/dL |
| IgE | 11 | mg/dL |
The patient’s results indicate elevated inflammatory markers, specifically C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). The Nitroblue Tetrazolium Test (NBT) is within normal limits. However, immunoglobulin levels (IgG, IgA, and IgM) are significantly low.
Initially, the treatment for suspected osteomyelitis included clindamycin, ceftriaxone, prednisolone, and ibuprofen. Following an immunology consultation, a localized purulent collection and neutropenia were identified, along with elevated phosphorus levels. With a suspicion of Hyperphosphatemic Tumoral Calcinosis, the treatment plan was revised on October 15, 2023, replacing prednisolone with colchicine and adding Sevelamer, acetazolamide, and antacids.
The child experienced vomiting and diarrhea, which were effectively managed with treatment. Due to the guarded prognosis, the patient was discharged in good general condition on October 19, 2023, with instructions to continue Sevelamer (800 mg daily) and to follow up in one month to monitor calcium, phosphorus, and vitamin D levels.
Currently, the patient’s overall health remains poor, with intermittent relapses of symptoms that fluctuate over time. These symptoms are partially controlled through biochemical management, with some improvement. The patient is not receiving Anakinra due to multiple side effects. Since there is no definitive cure for the condition, the focus of management is on controlling symptoms, preventing further complications, and improving quality of life. Despite the disease’s typical resistance to treatment, the patient has shown significant improvement, particularly with acetazolamide.
3. Discussion
The one-year-old patient exhibits clinical symptoms, including intermittent fevers, a growing mass in the gluteal region, and a limp, which are consistent with HFTC. The fevers may result from inflammation caused by calcified deposits in soft tissues, which are characteristic of HFTC. The mass in the gluteal region suggests ectopic calcification due to abnormal phosphate metabolism, leading to discomfort and restricted movement. Blood tests show elevated phosphorus and near-normal high calcium levels, further supporting the HFTC diagnosis. Additional findings, such as elevated alkaline phosphatase, slight acidosis, oxygenation issues, anemia, and abnormal blood counts, suggest potential bone, liver, or hematologic involvement. Elevated inflammatory markers (CRP, ESR) and low immunoglobulin levels indicate an inflammatory response and possible immunodeficiency. Overall, the symptoms and lab results point toward HFTC, potentially complicated by other conditions, necessitating further evaluation and management.
Hyperphosphatemic familial tumoral calcinosis is a genetic disorder characterized by painful calcifications around the joints, caused by mutations affecting phosphate metabolism. Key findings include elevated serum phosphate, normal or high 1,25-dihydroxyvitamin D, and increased PTH levels due to resistance to fibroblast growth factor 23 (FGF23). Unlike nephrologic diseases, which involve kidney-related phosphate and calcium balance issues, and rheumatologic diseases, which focus on joint inflammation and autoimmune processes, HFTC is marked by specific genetic mutations and periarticular calcifications (7).
Conditions such as chronic kidney disease (CKD), renal tubular acidosis (RTA), vitamin D deficiency, and hyperparathyroidism each have distinct symptoms and lab findings, differing from the genetic basis of HFTC (8). Rheumatologic diseases, such as systemic lupus erythematosus (SLE) and calcinosis cutis, present with joint pain, skin rashes, and positive autoantibodies, unlike HFTC, which involves genetic mutations and elevated phosphate levels without an autoimmune profile (7).
Differential diagnoses such as Fibrodysplasia Ossificans Progressiva (FOP), Calciphylaxis, and chronic inflammatory conditions differ from HFTC by their clinical presentation and underlying causes, highlighting the importance of specific diagnostic tools such as imaging and genetic testing for accurate diagnosis and management of HFTC (7, 8).
This case report highlights the use of Sevelamer, a phosphate binder, in treating HFTC, aligning with existing management strategies (1-4). Sevelamer binds dietary phosphate in the gastrointestinal tract, thereby reducing blood phosphate levels and potentially slowing the progression of calcification. The literature emphasizes the need for further research to assess the efficacy of treatment for HFTC (3).
Our study observed clinical and laboratory features, such as pain, hard masses, and elevated blood phosphorus levels, consistent with previous reports by Iwasaki (2023), Saifuddin (2023), and Fabbriciani (2024) (9-11). Laboratory findings typically show elevated phosphate levels with normal calcium and parathyroid hormone (PTH) levels, and occasionally elevated C-reactive protein (CRP), corroborating the findings of Fabbriciani (2024) and Iwasaki (2023) (9, 10). Management in our case involved dietary phosphate restriction and phosphate-binding agents like aluminum hydroxide and Sevelamer, in line with the recommendations of Saifuddin (2023) (11).
3.1. Limitations
As with any single case report, the findings cannot be generalized to the entire HFTC population. Furthermore, the absence of genetic testing information in this case limits a more comprehensive understanding of the underlying cause specific to this patient.
3.2. Conclusions
This case report provides important insights into HFTC through the presentation of a unique case involving a young child. The patient's clinical presentation, laboratory findings, and treatment approach align with the established characteristics of HFTC, underscoring the essential role of genetic testing and the ongoing need for research to develop more effective treatments for this rare disorder.
Early diagnosis and intervention are critical for improving outcomes and reducing complications, as timely detection can prevent the formation of calcific tumors in soft tissues, minimize pain and discomfort, and enhance quality of life. Early treatment also helps protect tissues and organs, maintains mobility, reduces the risk of infections, and manages elevated blood phosphate levels, thereby preventing complications from hyperphosphatemia.
In conclusion, prompt diagnosis and management of HFTC are crucial for improving patient quality of life, minimizing disease-related complications, and increasing life expectancy.