1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA virus that causes coronavirus disease 2019 (COVID-19) in humans. The virus can affect various organs, including the lungs, heart, ileum, kidneys, and bladder (1, 2). Acute COVID-19 infection is associated with widespread inflammatory processes and cytokine storm, which may lead to multiorgan failure (3). Multisystem inflammatory syndrome in children (MIS-C) is a rare complication of COVID-19, with an incidence rate of approximately 1% in children affected by COVID-19 (4). Multisystem inflammatory syndrome in children typically presents 4 to 6 weeks after the initial infection, and most patients have had mild or asymptomatic COVID-19. It is believed that MIS-C results from a delayed immune response (5, 6). The diagnostic criteria for MIS-C include: Age under 21 years, fever, inflammation involving two or more organ systems based on laboratory findings (e.g., cardiac, renal, respiratory, hematologic, gastrointestinal, cutaneous, or neurological), severe disease requiring hospitalization, and the exclusion of other potential differential diagnoses (7).
To our knowledge, there have been no prior reports of severe bone marrow suppression as a manifestation of MIS-C. In this report, we present the case of a 10-year-old boy with bone marrow suppression due to MIS-C.
2. Case Presentation
A ten-year-old boy was referred to our hospital with complaints of ecchymosis around the eyelids, particularly the upper eyelids, and lethargy. It was reported that the ecchymosis had progressed over the past four days, accompanied by a history of fever, cough, bone pain, and lethargy lasting ten days. During an earlier evaluation, a complete blood count (CBC) test revealed mild thrombocytopenia, and conservative treatment was recommended (results are shown in Table 1). After a few days, the fever and cough resolved, but the patient remained lethargic. He had no significant past medical or hospitalization history.
| Variables | Values |
|---|---|
| First tests | |
| WBC (/mL) | 1300 |
| Hb (g/L) | 5 |
| PLT (/mL) | 25000 |
| LDH | 5200 |
| Urea (mg/dL) | 288 |
| Creatinine (mg/dL) | 5.5 |
| Uric acid (mg/dL) | > 18 |
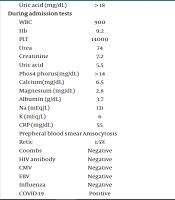
| During admission tests | |
| WBC | 900 |
| Hb | 9.2 |
| PLT | 14000 |
| Urea | 74 |
| Creatinine | 7.2 |
| Uric acid | 5.5 |
| Phosphorus (mg/dL) | > 14 |
| Calcium (mg/dL) | 6.5 |
| Magnesium (mg/dL) | 2.8 |
| Albumin (g/dL) | 3.7 |
| Na (mEq/L) | 131 |
| K (mEq/L) | 6 |
| CRP (mg/dL) | 55 |
| Prepheral blood smear | Anisocytosis |
| Retic | 1.5% |
| Coombs | Negative |
| HIV antibody | Negative |
| CMV | Negative |
| EBV | Negative |
| Influenza | Negative |
| COVID-19 | Positive |
| Last Tests | |
| WBC | 4700 |
| Hb | 10.5 |
| PLT | 241000 |
| LDH | 407 |
| Urea | 48 |
| Creatinine | 0.8 |
| Uric acid | 7 |
| Phosphorus | 4.1 |
| Calcium | 9 |
| Magnesium | 2.1 |
| Albumin | 4.2 |
| Na | 141 |
| K | 4 |
| CRP | 2 |
Laboratory Data
At the time of presentation, the patient was oriented to time and place, with no motor or neurological deficits, though he appeared markedly lethargic. His Glasgow Coma Scale (GCS) score was 14 - 15. His weight-for-age was above average, and both physical and neurological development were within normal limits. Laboratory tests revealed severe pancytopenia (WBC: 1300 /μL, Hb: 5 g/dL, PLT: 25,000 /μL). The patient did not exhibit active bleeding and denied any history of bleeding.
Biochemical studies demonstrated severe elevations in urea and creatinine levels, severe hyperphosphatemia, hyperuricemia, and electrolyte imbalances (urea: 250 mg/dL, creatinine: 5.5 mg/dL, hyponatremia sodium: 131 mEq/L, hyperkalemia potassium: 6.3 mEq/L, uric acid > 14 mg/dL, phosphorus > 14 mg/dL), indicating acute kidney injury (Table 1). Emergency hemodialysis was initiated due to these findings and the patient’s lethargy. Additionally, blood products, including packed red blood cells and platelet units, were transfused.
A brain CT scan was performed to investigate periorbital ecchymosis and lethargy, and the results were normal. At this stage, the patient had normal urine output, stable blood pressure, and no signs of volume overload. Physical examination of the lungs revealed slight tachypnea, and abdominal examination detected a palpable spleen. An abdominal ultrasound revealed enlarged kidneys (left: 118 mm, right: 115 mm), splenomegaly (150 mm), and a small amount of abdominal free fluid. A chest CT scan was also conducted and found to be normal.
Broad-spectrum antibiotics, including meropenem and vancomycin, were prescribed.
Rheumatologic diseases, including vasculitis and systemic lupus erythematosus (SLE), were ruled out due to normal rheumatologic laboratory tests, including ANA, anti-dsDNA, anti-Smith, antiphospholipid antibody, complement factors C3, C4, CH50, Coombs tests, P-ANCA, and C-ANCA. However, interleukin-6 and fibrinogen levels were significantly elevated. More importantly, the COVID-19 PCR test was positive, leading to the initiation of treatment with remdesivir (200 mg on the first day, followed by 100 mg for four days) and methylprednisolone pulse therapy (1 g per day for three days) based on the diagnosis of MIS-C. Other potential infectious diseases that could present with similar bone marrow suppression, such as leishmaniasis, were ruled out due to normal test results and a normal recent CBC test conducted a week before the onset of clinical manifestations.
Unfortunately, the patient developed hypertension, necessitating the prescription of antihypertensive drugs, including Metoral and Amlodipine. Intermittent hemodialysis was performed three times per week. Initially, the patient was anorexic, but after two sessions of hemodialysis, his appetite improved, and oral medications were initiated. Due to severe bone marrow suppression and the massive need for blood product transfusions, a bone marrow aspiration (BMA) was considered. However, based on a hematologic consultation, the risk of malignancy was deemed low due to peripheral blood smear findings, and the BMA was not performed due to the high risk of bleeding.
Subsequent laboratory test results revealed elevated levels of D-dimer and fibrinogen, further supporting the MIS-C diagnosis. Improvements in fibrinogen, C-reactive protein (CRP), D-dimer, urea, creatinine, and blood cell counts were observed by the fifth day of treatment, following the last dose of remdesivir. The patient continued dialysis every other day, with gradual normalization of uric acid, calcium, and phosphorus levels.
The laboratory tests showed improvement, although the CBC improved with a delay. At this point, hemodialysis was discontinued as the patient had normal urinary output. The patient was subsequently discharged with medical instructions, which included prescribed medications such as calcium carbonate tablets twice daily, allopurinol once daily, prednisolone 5 mg, amlodipine twice daily, and Metoral 25 mg daily. Weekly follow-up was recommended.
During the follow-up, laboratory results, including CBC and biochemistry tests, normalized. A follow-up ultrasound study reported normal kidney size. Antihypertensive medications and prednisolone were gradually tapered and discontinued within two months. The patient’s venous catheter was removed after one month.
3. Discussion
In this report, we presented a boy with MIS-C who did not exhibit fever. During evaluations, he was found to have pancytopenia, which worsened over time. The MIS-C typically occurs 3 to 6 weeks after COVID-19 infection and represents a post-infection hyperinflammatory response with varying symptoms and severity. The condition is characterized by persistent fever and involvement of two or more organ systems. Patients with MIS-C often report mild COVID-19 symptoms or may not recall any prior illness; however, a positive COVID-19 test usually indicates previous asymptomatic infection. Diagnosis is based on abnormal vital signs, elevated inflammatory markers, positive COVID-19 tests or exposure to confirmed cases, and the exclusion of other differential diagnoses. While gastrointestinal, skin, and neurological manifestations are common, severe hematologic manifestations are rare. Mild decreases in hemoglobin, reduced lymphocytes, and increased leukocytes (mainly neutrophils) are typically observed in MIS-C (8, 9).
Our patient had a history of fever, cough, bone pain, and lethargy, which were likely related to a previous COVID-19 infection. At the time of the visit, however, he did not have a fever, which could be explained by severe pancytopenia. Fever is one of the main symptoms of MIS-C (10, 11). Chinniah et al. reported that 100% of patients with MIS-C presented with fever (12), while Lampidi et al. found fever in 99% of their cases (13). Although MIS-C is a rare complication of COVID-19, with an incidence of approximately 45 to 54 cases per 100,000 COVID-19 infections (14, 15), cases without fever, such as ours, are extremely rare. La Torre et al. demonstrated that the diagnostic criteria for MIS-C are not always specific, and some cases may not fully meet the established criteria (16). This observation aligns with our case, highlighting the importance of physicians considering atypical presentations that may lack the standard diagnostic features of MIS-C.
The vast majority of patients with MIS-C exhibit inflammatory abnormalities in their blood tests. Commonly elevated inflammatory markers in MIS-C include procalcitonin, erythrocyte sedimentation rate (ESR), CRP, lactic acid dehydrogenase (LDH), D-dimer, and ferritin. Additionally, MIS-C is often associated with an elevated white blood cell (WBC) count accompanied by lymphopenia, as well as lower platelet counts (4, 17). In the laboratory studies of our case, there was severe pancytopenia (WBC: 900/mL, hemoglobin: 5 g/dL, platelets: 14,000/mL at the worst point), increased LDH, and evidence of renal failure, including very high levels of urea and creatinine, severe hyperphosphatemia, severe hyperuricemia, and electrolyte abnormalities. Based on our review, cases of MIS-C with severe pancytopenia are extremely rare.
Alrefaey et al. reported a similar case involving a 12-year-old girl with no prior medical history who presented with shortness of breath and dizziness over two days. A polymerase chain reaction (PCR) test confirmed COVID-19 infection. Laboratory tests showed elevated inflammatory markers, leukopenia (WBC: 2000/mL), and elevated liver enzymes (18). The patient had persistent fever with peaks reaching 40ºC, which did not respond to antibiotics or anti-inflammatory treatment. Intravenous immunoglobulin (IVIG) and steroids were prescribed initially but did not result in clinical improvement. However, after the addition of two corticosteroid pulses, the inflammatory markers decreased, and the patient’s condition improved (18).
In contrast to the case reported by Alrefaey et al., our patient exhibited even more severe bone marrow suppression, with a WBC count of 900/mL, and notably, did not have a fever. This highlights the exceptional severity of bone marrow suppression in our case, underscoring the atypical and severe hematologic manifestations of MIS-C (18).
The diagnosis of rheumatologic disorders, such as SLE and other vasculitides, had to be considered in this case because these conditions can mimic MIS-C (19). To rule out these possibilities, laboratory tests including antinuclear antibody (ANA), anti-double strand DNA, and other rheumatological markers were performed, all of which were reported negative. Additionally, the patient did not present with glomerulonephritis or hematuria, further reducing the likelihood of these differential diagnoses.
Although the most common treatments for MIS-C include methylprednisolone, intravenous immunoglobulin (IVIG), and remdesivir, the use of these therapies remains a topic of debate (20). Ichiyama et al. reported on the outcomes of a combination therapy involving remdesivir, tocilizumab, and methylprednisolone pulse, noting significant improvements in severe lung involvement associated with COVID-19 (21). Similarly, Mastruzzo et al. demonstrated that a combination of methylprednisolone pulse and remdesivir significantly improved outcomes in severe COVID-19 cases, including reduced mortality rates, improved oxygen saturation, and a decreased need for mechanical ventilation (22). However, there is limited data on the use of this combination specifically for MIS-C.
In this case, we administered a combination of methylprednisolone pulse and remdesivir and found it to be a rapid and effective treatment for a severe presentation of MIS-C.
3.1. Conclusions
Multisystem inflammatory syndrome in children is a rare complication of COVID-19, and severe bone marrow suppression is an exceptionally rare presentation. In patients with suspected MIS-C and COVID-19, even in the absence of fever, bone marrow suppression should be considered. Physicians may find the combination of remdesivir and methylprednisolone pulse to be a highly effective treatment for such cases.