1. Background
The Bacille Calmette-Guérin (BCG) vaccine has been used as an anti-tuberculosis (TB) vaccine since 1921, proving especially effective as a protective measure against TB meningitis and miliary TB (1). It is estimated that 100 million children worldwide and a quarter-million Iranian newborns receive the BCG vaccine annually (2). Based on recommendations from the Iranian National Advisory Committee on Immunization, newborns are administered the BCG vaccine at birth. This vaccine is generally considered safe, with serious vaccine-induced complications being very rare in the general population. Such complications include systemic or disseminated BCG disease (DBD), which primarily occurs in immunocompromised patients, with reported incidence rates of 1 in 230,000 to 640,000 vaccination cases (3). Manifestations of DBD include constitutional symptoms such as fever, weight loss, splenomegaly, hepatomegaly, sepsis-like signs, and multiple organ dysfunctions (4). Due to the lack of symptom specificity, a diagnosis requires a high level of clinical suspicion (5). Various criteria have been suggested for defining DBD (6-9). In this study, DBD classification was based on the criteria suggested by Bernatowska et al. (9).
As supported by various studies, approximately 90% of patients with DBD have an underlying immunodeficiency (4-6, 10, 11). The primary immunodeficiency conditions that predispose infants to this severe disease include severe combined immunodeficiency (SCID), chronic granulomatous disease (CGD), complete DiGeorge syndrome (cDGS), acquired immune deficiency syndrome (AIDS), and Mendelian susceptibility to mycobacterial diseases (MSMD) (12).
Given the rarity of DBD, most global reports are presented in the form of case studies, resulting in limited evidence regarding the immunodeficiencies associated with the disease, treatment protocols, and follow-up strategies for these children.
2. Objectives
The aim of this study was to determine the epidemiological, clinical, and immunological characteristics of patients with DBD based on standard diagnostic criteria. In addition, we evaluated treatment regimens, survival, and prognosis for each patient.
3. Methods
3.1. Study Setting and Patients
In this longitudinal cross-sectional study, participants were selected from children referred to Alzahra and Imam Hossein Hospitals in Isfahan, Iran. These hospitals are major tertiary referral centers for Isfahan Province, located in central Iran, with a population exceeding 5 million. Files from infants and children diagnosed with DBD between 2005 and 2017 were reviewed, and relevant data were collected. A total of 22 patients were included in this study, with no exclusions. The Ethics Committee of Isfahan University of Medical Sciences approved the study protocol (IR.MUI.REC.1395.3.822).
3.2. Eligibility Criteria
The inclusion criteria required obtaining informed consent from the patients' parents and a confirmed diagnosis of DBD based on the criteria mentioned earlier. Exclusion criteria included incomplete patient files as per their admission process, any inflammation lacking typical histopathologic changes, or the inability to isolate Mycobacterium tuberculosis complex (MTBC) by polymerase chain reaction (PCR) in patients with primary immunodeficiency. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences.
3.3. Data Gathering
All information regarding patients’ clinical presentations, laboratory data, radiologic findings, Mycobacterium bovis evaluations, immune system assessments, and patient outcomes was thoroughly extracted from their profiles. For most children, immunological evaluations were performed during their initial hospitalization, with further investigations completed later for some patients who required additional assessment.
Data regarding the treatment regimens, including medication names, treatment duration, and any potential drug-related side effects, were gathered from their medical records.
For all patients suspected of having DBD, bone marrow aspiration and gastric lavage and/or tracheal aspirate samples were obtained. All bone marrow aspiration or biopsy samples were evaluated for typical histopathologic features of mycobacterial infections, such as granulomatous inflammation with caseous necrosis. Additionally, acid-fast bacilli positivity in smears and histological specimens of bone marrow, gastric lavage, and/or tracheal aspirate samples was assessed. The PCR technique was employed for detecting MTBC or Mycobacterium bovis BCG substrain whenever feasible. Mycobacterium bovis BCG was differentiated from other members of the M. tuberculosis complex through standard PCR amplification across the junctions of the region of difference RD1 (13). Aliquots for PCR analysis were obtained directly from the patient samples. Strict procedures and controls were implemented to prevent cross-contamination in mycobacterial PCR (14). “Bacille Calmette-Guérin isolated” was defined as the isolation of M. bovis BCG by PCR; “M. tuberculosis isolated” was defined as the isolation of M. tuberculosis by PCR.
For each patient, immunodeficiency was evaluated according to Bonilla et al. and Oliveira and Fleisher, which enabled the diagnosis of the underlying immunological condition in most cases (15, 16).
4. Results
Twenty-two patients were eligible for this study. Fifteen patients (61.8%) were male, 2 patients (9%) had a family history of immunodeficiency, and 10 patients (45.5%) had parental consanguinity. Patients diagnosed with SCID had a mean age of 5.5 months, while children diagnosed with MSMD had a mean age of 2.94 months (excluding patient number 11 due to skewing) at the time of their initial presentation of the disease.
We have organized our findings into five categories based on modality: Clinical manifestations; laboratory data and radiologic findings; M. bovis evaluations; immune system evaluations; and patient outcomes.
4.1. Clinical Manifestations
The most common presenting symptoms among patients with DBD were fever (65.3%), axillary lymphadenitis (30.7%), and weight loss (26.9%). One patient (3.8%) presented with parotid and submandibular lymphadenopathy (LAP), and another (3.8%) had cervical LAP. At the time of diagnosis with BCG disease, two patients (7.69%) exhibited sepsis, three (11.5%) had diaper candidiasis, one had chronic diarrhea, and ten had oral candidiasis. No significant past medical history was recorded for the patients, except for one 5-year-old who had presented with impetigo at 3 months, axillary lymphadenitis at 6 months, and subsequently developed septic arthritis and Henoch-Schönlein purpura at age 3. Eleven patients (42.3%) were reported to have parental consanguinity. Two patients (7.69%) had a positive family history of immunodeficiency, and another two (7.69%) had an indefinite family history.
4.2. Laboratory Data and Radiologic Findings
In abdominal ultrasound studies, six patients were reported as normal. Nineteen patients presented with hepatosplenomegaly (with it being the only finding in seven of these cases), eight had para-aortic LAP (36.3%), three had ascites and free fluid (13.6%), two had portohepatic LAP (9%), two showed hypo-echoic lesions in the liver suggestive of TB (9%), and one patient presented with lesions suggestive of liver candidiasis. The tuberculin skin test was negative in all patients.
In chest X-ray studies, 14 patients (63.6%) showed no significant findings, while 9% exhibited infiltrations. The thymus shadow was absent in 9%, two patients (9%) had a small thymus, one patient (4.5%) had pleural effusion, and 9% presented with signs of pneumonia.
In computerized tomography (CT) scan results, 9% of patients presented with para-aortic and portohepatic LAP, and one patient had ascites. A total of 77.2% of the patients were reported as normal with no significant findings.
In CBC findings, 17 patients presented with anemia (77.2%), 13.6% had leukopenia, 27.2% had leukocytosis, 9% had thrombocytopenia, 31.8% had thrombocytosis, and 22.7% had lymphopenia.
Seven patients (31.8%) had abnormal liver transaminase levels, 90.9% had elevated ESR and/or CRP levels, 18% showed elevated LDH levels, one patient's lab results suggested kidney dysfunction (4.5%), 13.6% had hyponatremia, and low albumin levels were observed in 41% of the patients.
Each of the 22 patients presented with a unique pattern of laboratory findings. In the following paragraph, we present the prevalence of abnormalities in each laboratory test.
4.3. Mycobacterium bovis Evaluations
Of the patients, 54.5% underwent gastric lavage smear, with 40.9% of these yielding positive results (one patient had a negative smear but a positive culture).
Among the 17 patients who underwent bone marrow aspiration sampling, 22.7% were smear-negative, 18.1% were culture-positive, 18.1% were PCR-positive, 9% were AFB-positive, one patient (4.5%) was smear-positive, 4.5% showed a T-cell drop, one patient was AFB-negative, and one was PCR-negative.
Other sporadic methods for isolating TB bacillus included: Ascites fluid culture and smear, colon biopsy culture and smear, abscess aspiration AFB test and PCR (in two patients), peripheral blood PCR showing positive AFB in one patient, AFB-positive tracheal sample, and positive ulcer biopsy, each carried out in one patient.
4.4. Immune System Evaluations
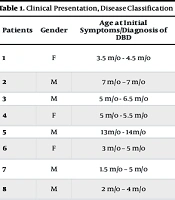
Almost all patients (except patient number 21) were evaluated according to the proposed protocol as previously mentioned. Of the remaining 20 patients, seven had SCID, ten had IL-12Rβ1 deficiency, and one had Wiskott-Aldrich syndrome (WAS). No known immunodeficiency was detected in three of the patients. Further details are shown in Table 1.
| Patients | Gender | Age at Initial Symptoms/Diagnosis of DBD | Systemic Syndrome (Clinical Presentation) | Mycobacterium Isolation (Site/Method) | DBD Classification | Immunodeficiency Type |
|---|---|---|---|---|---|---|
| 1 | F | 3.5 m/o - 4.5 m/o | Fever, rash, cough, LRTI, HSM, LAP (axillary, para-aortic), diffuse alveolar opacity on CXR | BMA positive (AFB smear) | Possible DBD | SCID |
| 2 | M | 7 m/o - 7 m/o | Fever | GA positive (AFB smear) + NGI in LN biopsy | Possible DBD | Unidentified immunodeficiency |
| 3 | M | 5 m/o - 6.5 m/o | Fever, HSM, LAP (axillary) | BMA positive (AFB smear + NGI) | Possible DBD | SCID |
| 4 | F | 5 m/o - 5.5 m/o | HSM, LAP (axillary, portohepatis, mesenteric) | GA positive (AFB smear) | Possible DBD | Unidentified immunodeficiency |
| 5 | M | 13 m/o - 14 m/o | Fever, HSM, LAP (axillary) | BMA positive (AFB smear + NGI) | Possible DBD | WAS |
| 6 | F | 3 m/o - 5 m/o | HSM, LAP (axillary, para-aortic, portohepatis) | GA & BMA positive (culture & PCR) (BCG isolated) | Definitive DBD | IL-12Rβ1 deficiency |
| 7 | M | 1.5 m/o - 5 m/o | HSM, LAP (axillary, inguinal) | GA positive (PCR) (MTBC is isolated) | Probable DBD | IL-12Rβ1 deficiency |
| 8 | M | 2 m/o - 4 m/o | Fever, WL, cough, chronic diarrhea, LRTI, HSM, lobar consolidation on CXR | BMA positive (AFB smear) | Possible DBD | SCID |
| 9 | F | 4 m/o - 11 m/o | Fever, WL, LRTI, HSM, LAP (axillary, abdominal), ascites, miliary opacification on CXR | GA & BMA positive (culture & PCR) (BCG isolated) | Definitive DBD | IL-12Rβ1 deficiency |
| 10 | M | 5 y/o - 5 y/o | Fever, LAP (axillar, para-aortic, cervical, portohepatic) | GA positive (culture & PCR) (BCG isolated); colon biopsy positive (AFB smear + NGI) | Definitive DBD | IL-12Rβ1 deficiency |
| 11 | M | 18 m/o - 18 m/o | HSM, LAP (axillary, cervical, para-aortic) | GA & BMA positive (PCR) (BCG isolated) | Definitive DBD | Tyk2 deficiency |
| 12 | M | 4 m/o - 9 m/o | Fever, WL, HSM, LAP (axillary, cervical, submandibular), ascites, multiple hypoechoic lesions in spleen | GA positive (AFB smear) | Possible DBD | IL-12Rβ1 deficiency |
| 13 | M | 4 m/o - 4 m/o | Fever, HSM, LAP (axillary), jaundice, ascites, diffuse alveolar opacity on CXR | GA & BMA positive (PCR) (MTBC is isolated) | Possible DBD | IL-12Rβ1 deficiency |
| 14 | F | 3 m/o - 5 m/o | Fever, WL, HSM, LAP (axillary, para-aortic, mesenteric), ascites | GA & BMA positive (PCR) (MTBC is isolated) | Probable DBD | IL-12Rβ1 deficiency |
| 15 | F | 3 m/o - 7 m/o | Fever, HSM, LAP (axillary, cervical, para-aortic) | GA & BMA positive (culture & PCR) (BCG isolated) | Definitive DBD | IL-12Rβ1 deficiency |
| 16 | M | 3 m/o - 4 m/o | Fever, WL, HSM, multiple hypoechoic lesions in spleen | GA & BMA positive (PCR) (MTBC is isolated) | Probable DBD | SCID |
| 17 | M | 3 m/o - 4 m/o | Fever, HSM, LAP (axillary), multiple hypoechoic lesions in spleen | BMA positive (PCR) (MTBC is isolated) | Probable DBD | SCID |
| 18 | M | 4 m/o - 4 m/o | Fever, WL, cough, LRTI, HSM, LAP (axillary) , diffuse alveolar opacity on CXR | BMA positive (PCR) (MTBC is isolated) | Probable DBD | SCID |
| 19 | M | 3 m/o - 3 m/o | Fever, cough, LRTI, HSM, ascites, multiple hypoechoic lesions in liver, bronchopneumonia on CXR | GA positive (AFB smear) | Possible DBD | SCID |
| 20 | F | 2 m/o - 3.5 m/o | Fever, WL, HSM, LAP (axillary) | GA & BMA positive (PCR) (MTBC is isolated) | Probable DBD | IL-12Rβ1 deficiency |
| 21 | M | 2 m/o - 2 m/o | Fever, splenomegaly, LAP (axillary, cervical, inguinal) | GA & BMA positive (AFB smear) | Possible DBD | Unidentified immunodeficiency |
| 22 | M | 3 m/o - 3 m/o | Fever, WL, HSM, LAP (axillary, para-aortic) | GA positive (AFB) BMA positive (AFB smear + NGI) | Possible DBD | IL-12Rβ1 deficiency |
Abbreviations: AFB, acid-fast bacilli; ALRTI, acute lower respiratory tract infection (tachypnea or chest indrawing) (17); BMA, bone marrow aspiration; BCG, Bacille Calmette-Guérin; DBD, disseminated BCG disease; F, female; GA, gastric aspiration; HSM, hepatosplenomegaly; LAP, lymphadenopathy; M, male; m/o, months old; MTBC, M. tuberculosis complex; NGI, necrotizing granulomatous inflammation; PCR, polymerase chain reaction; WL, weight loss; SCID, severe combined immunodeficiency.
4.5. Patient Outcome
Based on the suggested diagnostic criteria for DBD in children with primary immunodeficiency (8), the patients with DBD were categorized into three groups: Definitive, probable, and possible DBD. Further details are shown in Table 1.
Table 2 presents treatment details for each of the 22 patients, along with comorbidities, type of immunodeficiency (if known), patient outcomes, and attributed causes of death. Treatment involved initiating at least four anti-TB drugs, excluding pyrazinamide due to M. bovis resistance. Given the limitations in our country regarding evaluations for the gamma-IFN/IL-12 pathway, and the fact that a significant percentage of patients with DBD have an underlying MSMD immune condition, we began an empirical treatment with recombinant interferon gamma for patients without specific findings of immunodeficiency. This was administered at a dosage of 100 - 200 micrograms/m² via subcutaneous injection three times a week, alongside anti-TB therapy. All children were regularly monitored for treatment complications. Except for those diagnosed with SCID, who succumbed to causes beyond drug treatment, all surviving patients tolerated the drug regimen well, with no severe drug complications observed.
| Patients | Treatment (Duration) | Additional Sign & Symptom (Probably Unrelated to BCG Vaccination) | Outcome | |
|---|---|---|---|---|
| Initiation Phase | Continuation Phase | |||
| 1 | RHEClr | - | - | Died at that hospitalization (4 m/o) |
| 2 | RHEClr/IFN-γ | - | - | Died at 13 m/o |
| 3 | RHAzm | - | Diaper and oral candidiasis | Died at 9 m/o |
| 4 | RHAzm | - | Diaper and oral candidiasis | Unknown |
| 5 | RHEClr/IFN-γ (2 y) | HE IFN-γ (until death) | He developed acute rejection after receiving BMT at age of 6 m/o that recovered but had repeated episodes of recurrent infection | Died at 6 y/o after 2nd BMT |
| 6 | RHEClr/IFN-γ (4 m) | RH IFN-γ (8 m) | Oral candidiasis; palpable erythematous maculopapular skin lesions with arthritis (twice, improved spontaneously) | Alive 9.5 y/o |
| 7 | RHEClr/IFN-γ (4 m) | RH IFN-γ (8 m) | Oral candidiasis; palpable erythematous maculopapular skin lesions with arthritis improved with antibiotics at age of 8 y/o | Alive 8.5 y/o |
| 8 | RHEClr/IFN-γ | - | Oral candidiasis | Died at 6 m/o |
| 9 | RHEClr/IFN-γ (4 m) | RH IFN-γ (8 m) | Oral candidiasis | Alive 9.5 y/o |
| 10 | RHEClrAMK/IFN-γ (6 m) | RH IFN-γ (12 m) | Oral candidiasis | Alive 16 y/o |
| 11 | RHEClr/IFN-γ (3 m) | RH IFN-γ (9 m) | Oral candidiasis; allergic symptoms (rhinitis & Skin) | Alive 5.5 y/o |
| 12 | RHEClr/IFN-γ (2 m) | - | - | Died at 11 m/o |
| 13 | HEClrAMK/IFN-γ | - | - | Died at that hospitalization (4 m/o) |
| 14 | RHEClr/IFN-γ (4 m) | RH IFN-γ (9 m) | Oral candidiasis; repeated episodes of palpable erythematous maculopapular skin lesions with severe arthritis (leukocytoclastic vasculitis on skin biopsy with positive blood culture for salmonella at first presentation and improved with antibiotics), next episodes controlled with oral antibiotics | Alive 5.5 y/o |
| 15 | RHEClr/IFN-γ (3 m) | RH IFN-γ (9 m) | Oral candidiasis; repeated episodes of palpable erythematous maculopapular skin lesions with severe arthritis (leukocytoclastic vasculitis on skin biopsy with negative cultures of blood and stool for salmonella) that improved with antibiotics), next episodes controlled with oral antibiotics | Alive 8.5 y/o |
| 16 | RHEClr/IFN-γ (4 m) | RH (so far) | Disseminated candidiasis | Alive 3 y/o |
| 17 | RHEClr/IFN-γ | - | Diaper candidiasis; sepsis | Died at that hospitalization (4 m/o) |
| 18 | RHEClr | - | Diaper candidiasis; sepsis | Died at that hospitalization (4 m/o) |
| 19 | RHEAzm | - | Oral candidiasis, bilateral nephrocalcinosis | Died at that hospitalization (3 m/o) |
| 20 | RHEClr/IFN-γ (5 m) | RH-IFN-γ (9 m) | Oral candidiasis; bilateral nephrocalcinosis at infancy that improved with conservative treatments | Alive 8 y/o |
| 21 | RHEClr/IFN-γ (3 m) | RH-IFN-γ (9 m) | - | Alive 16 m/o |
| 22 | RHEAm (1 y) | R ± H (so far) | Oral candidiasis; cutaneous leishmaniasis at age 6 years old for 2 years duration resistant to that cured with interferon gamma; repeated episodes of recurrent bacterial infection; developed muscle weakness since 7 years old (positive genetic testing for Duchenne muscular dystrophy) | Alive 15 y/o, bedridden |
Abbreviations: Am, amikacin; Azm, azithromycin; BMT, bone marrow transplantation; Clr, clarithromycin; E, ethambutol; H, isoniazid; IFN-γ, interferon gamma; m/o, months old; R, rifampicin; SCID, severe combined immunodeficiency; WAS, Wiskott-Aldrich syndrome; y/o, years old.
All SCID patients died due to sepsis. Among patients with MSMD, two died: One due to poor treatment compliance and another from gram-negative sepsis. Our patient with WAS died from complications related to bone marrow transplantation.
5. Discussion
In the present study, 22 children who were brought to one of two referral pediatric hospitals in Isfahan over a 13-year period were evaluated. Most of the patients were immunocompromised, with each condition calculated as follows: 26.9% of patients had SCID, 38.4% (12 patients) had MSMD (mainly IL-12Rβ1 deficiency), and one patient had WAS. The best prognosis was observed in MSMD patients with a four-drug regimen.
Disseminated BCG disease is a life-threatening complication most commonly seen in immunocompromised children. Various criteria have been proposed for defining DBD (6-9). These definitions primarily focus on the isolation of M. bovis or the identification of histopathological evidence of mycobacterial infection in one or more anatomical areas outside the vaccination site (such as lymph nodes beyond the vaccination site, respiratory secretions, bone marrow specimens, peritoneal fluid, etc.), along with clinical signs and symptoms consistent with DBD (e.g., fever, weight loss, and failure to thrive). The diagnosis is classified as definitive, probable, or possible, depending on whether the M. bovis BCG vaccine strain was detected by serological tests, PCR, or culture, and whether typical histopathological changes and granulomatous inflammation are present without microbial isolation (6-9).
Currently, no treatment guidelines are available for BCG-induced systemic complications, and anti-mycobacterial drugs have been used in various protocols. Treating the underlying disease in immunocompromised patients has also been recommended. Despite adequate treatment, mortality rates remain high, reported between 25% and 80% (6-9, 18-23). Factors such as lymphadenitis and injection site abscess, along with other elements like BCG strain type, physical-chemical properties, bacillary load, and administration method, influence the development of non-serious complications after BCG vaccine injection. However, systemic complications are primarily seen in immunocompromised children (23-25).
In this study, 22 children who were admitted to one of two referral pediatric hospitals in Isfahan over a 13-year period were evaluated. Although hospital-based studies have limitations, they can be valuable in the absence of extensive population-based studies for evaluating manifestations, treatment approaches, and prognosis of BCG-induced diseases. All of our patients were assessed for the presence of immune compromise.
In studies reporting signs and symptoms of BCG-induced disseminated disease, the major symptoms include coughing (72%), pyrexia (61%), anorexia and weight loss (40%), and diarrhea and vomiting (33%). The most frequent clinical signs observed are hepatomegaly (82%), splenomegaly (54%), and adenopathies (46%) (6, 26). Findings from our study align with these observations. Our experience from areas where BCG vaccination is routine as a prophylactic measure suggests that in every infant presenting with prolonged fever, weight loss, failure to thrive, skin rashes, hepatomegaly, or splenomegaly, BCG-induced disseminated disease should be considered.
Since DBD lacks specific manifestations and is a rare disease, it is often not evaluated in affected children. Additionally, in many developing countries, including Iran, determining the bacilli strain is not feasible in most medical centers (27). To detect Mycobacterium, gastric aspirate specimens and bone marrow biopsy samples are routinely collected from all suspected patients. Evaluations recommended by Hesseling et al. (7) were completed for all patients, with results presented in the tables above.
Despite the recommendations from Hesseling et al. (7), and recognizing that in infants who have received the BCG vaccine, Mycobacterium bacilli may be present in lymph node specimens from the inoculation area, we do not use a biopsy from that area to confirm the diagnosis.
Various studies have reported inconsistent information on the type of immune deficiency and the percentage of patients with BCG disseminated disease. In earlier reports, nearly 50% of patients did not have a diagnosed immune deficiency, although they “seemed to have some dysfunctions in their immune system” (28). In the study reported by Lotte et al., two-thirds of patients with BCG disseminated disease from 1921 to 1977 had immune deficiencies (21). The immune deficiencies most frequently associated with BCG disseminated disease are primary immunodeficiencies such as SCID, CGD, cDGS, and MSMD (i.e., disorders of the gamma-IFN/IL-12 pathway), and acquired immune deficiencies such as AIDS (9, 10, 20, 29-35).
In our study, most patients were immunocompromised, with the following percentages for each condition: 26.9% of patients had SCID, 38.4% (12 patients) had MSMD (mainly IL-12Rβ1 deficiency), one patient had WAS, and the rest had an unknown type of immunodeficiency. All children with leukopenia or lymphopenia were tested for HIV, and none were found to be HIV-positive. It is important to note that since this study’s population was not sampled from the community, we cannot use the percentages of each immunodeficiency type to determine the level of susceptibility to DBD for each type.
Given the pattern of M. bovis microbial resistance and the poor prognosis for children who remain untreated or inadequately treated with anti-TB medications (6, 36, 37), most guidelines recommend treatment with at least four anti-TB drugs (excluding pyrazinamide due to M. bovis resistance) for a minimum of nine months, depending on the patient’s response, followed by a prophylactic regimen until the immunodeficiency is resolved (7-9, 38-40).
We adhered to these recommendations with our patients, and except for those with SCID, all had a favorable prognosis. For these patients, we used a four-drug regimen consisting of isoniazid, rifampicin, ethambutol, and a novel macrolide (clarithromycin or azithromycin), which are well tolerated in children and have fewer complications than quinolones. After 3 to 4 months, based on the patients' clinical response, isoniazid and rifampicin were continued for at least a year. Patients with MSMD deficiency showed no signs of recurrence, despite the absence of prophylactic administration during their follow-up, which, for some, extended over 10 years.
As mentioned earlier, despite appropriate treatment, the mortality rate for children with DBD is as high as 85%. However, Lu et al. suggests that the prognosis for these patients depends significantly on their underlying immunodeficiency (27). Unfortunately, all children in our study with SCID as an underlying condition succumbed to the disease. The other child who died in our study was a patient with WAS, who passed away due to acute rejection following a second unsuccessful bone marrow transplant. On the other hand, all 10 patients with MSMD were successfully treated and, despite discontinuing anti-TB medications, did not experience relapse throughout the entire follow-up period. We believe this outcome should be considered in the treatment of such patients, as some practitioners may not adequately account for the more favorable prognosis in MSMD cases due to generally poor prognostic reports for patients with disseminated infections, while the response and long-term prognosis in MSMD patients appear satisfactory.
5.1. Limitations
This study has several strengths, including strict inclusion criteria, comprehensive clinical and immunological information, a nearly uniform treatment method for all patients, close follow-up of surviving children for up to 12 years, and the recording of data unrelated to BCG as well. These aspects are notable strengths of the present study. However, as a hospital-based study conducted at two main referral children's hospitals, it also has some limitations, as with any study. Firstly, since this study is based on patient referrals, we cannot draw any conclusions about the prevalence of such complications within the general population.
5.2. Conclusions
Infections caused by BCG are under-reported in developing countries. Since these complications are relatively rare and the clinical manifestations of disseminated infection resemble those of sepsis, diagnosing systemic infections caused by the BCG vaccine in children requires a high degree of clinical suspicion and the use of appropriate diagnostic measures, such as culturing mycobacterium and performing biochemical speciation or PCR. These measures should be taken promptly in cases of suspected DBD, and, if confirmed, the underlying immunodeficiency should be identified and appropriate treatments initiated where possible.
All major complications caused by the BCG vaccine should be reported to the Adverse Event Following Immunization (AEFI) committee, so that collected information can be reviewed and synthesized to improve diagnostic and therapeutic approaches. We recommend the four-drug treatment protocol, as it results in a satisfactory clinical response and leaves minimal sequelae in MSMD patients.