1. Introduction
Cardiac implantable electronic devices (CIEDs), including pacemakers and implantable cardioverter-defibrillators (ICDs), are essential for managing cardiac rhythm disorders but carry significant risks, such as infections, particularly in patients with chronic kidney disease (CKD) or end-stage renal disease (ESRD) undergoing hemodialysis (1). Their use has dramatically increased over the past several years, largely due to expanded indications from large clinical trials and the aging population (2). The CIEDs have evolved from single-chamber fixed-rate devices to advanced dual-chamber rate-responsive cardioversion and defibrillation systems, increasingly replacing older models (3). Implantable medical devices (IMDs) are vital in modern healthcare, enhancing survival and quality of life across various applications. With their increasing use, ensuring safety, efficacy, and cost-effectiveness has become a priority, driving efforts to improve regulations and patient outcomes (4).
While lifesaving, CIEDs are associated with complications such as infections, device failures, lead dislodgement, arrhythmias, and psychosocial issues like anxiety and depression (5). Additionally, it is crucial to recognize that these devices’ complexity and potential for failure necessitate vigilant monitoring and management to mitigate associated risks (6). Acute infections may occur intraoperatively or shortly after, requiring device removal and antibiotic therapy. Chronic infections can manifest months later, involving low-virulence organisms (5). Infections can range from localized pocket infections to systemic infections such as endocarditis, often caused by gram-positive bacteria like coagulase-negative staphylococci and Staphylococcus aureus, which are highly adherent to non-biological materials such as device leads and generators (7). The CIED infections can result in high mortality rates, reported to reach up to 18%, highlighting their severe impact on patient outcomes (8).
Recent research highlights the critical role of intensifying perioperative prophylaxis to prevent CIED infections. The PADIT trial found that while an incremental bundle of antibiotics did not significantly lower overall infection risk, it altered the infection microbiology, suggesting the need for alternative preventive strategies (9). The 2023 American Heart Association update further underscores the necessity of tailored perioperative antibiotic prophylaxis for high-risk patients (10). Moreover, a 2024 study emphasizes that bloodstream infections can cause severe complications, reinforcing the urgency for enhanced prophylactic measures to prevent device-related infections (11). In this case report, we present a 6-year-old boy with a history of tetralogy of Fallot (TOF) repair and recurrent CIED infections, demonstrating the critical importance of timely intervention and comprehensive management strategies.
2. Case Presentation
2.1. History and Physical Examination
A 6-year-old male was referred to Rajaei Heart Hospital, a tertiary pediatric cardiac center, with recurrent episodes of refractory fever. He presented with persistent erythema and swelling at the site of his epicardial permanent pacemaker (PPM). His medical history was significant for TOF, which had been surgically repaired at 18 months of age. Following the repair, an epicardial PPM was implanted due to complete heart block.
Approximately two months after the initial PPM placement, the patient developed localized erythema, swelling, and purulent discharge at the pacemaker pocket in the abdominal region, raising suspicion of a CIED pocket infection. Antimicrobial therapy was initiated, and the infected generator was removed. A new dual-chamber PPM was implanted in the left subclavian region. The patient remained asymptomatic until six months prior to his current presentation, when trauma to the left subclavian region resulted in a fractured ventricular lead. This necessitated the insertion of a new ventricular lead along with two additional leads.
Three weeks after the lead replacement, the patient returned with fever and localized symptoms. Physical examination revealed a febrile child with a tender, 3 × 4 cm swelling at the PPM pocket. There were no systemic signs of infection or hemodynamic instability. Despite initial interventions, the patient presented again three weeks later with persistent fever and headache. On examination, he appeared ill but was non-toxic, with stable hemodynamic parameters: Blood pressure of 105/75 mmHg, heart rate of 130 beats per minute, respiratory rate of 26 breaths per minute, and axillary temperature of 39°C. He was alert and responsive to verbal and painful stimuli. Localized examination showed tenderness and swelling at the PPM pocket site, measuring 3 × 4 cm. Systemic examination, including cardiovascular and respiratory assessments, was otherwise unremarkable. These findings necessitated further diagnostic evaluation and planning for management of the suspected recurrent CIED infection.
3. Method
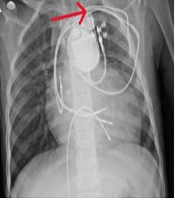
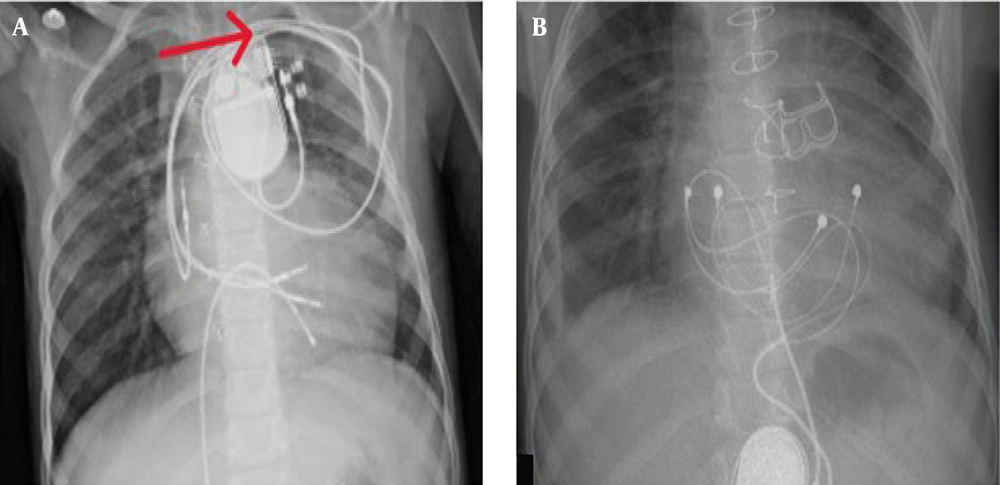
A provisional diagnosis of CIED infection prompted a comprehensive diagnostic workup. Laboratory evaluation revealed leukocytosis with neutrophilia on complete blood count (CBC), and blood cultures grew coagulase-positive S. aureus (Table 1). A chest X-ray showed no evidence of pulmonary complications (Figure 1). Transesophageal echocardiography (TEE) ruled out the presence of vegetation on the device leads or cardiac structures.
| Laboratory Index | Patient | Normal Range |
|---|---|---|
| Hemoglobin (g/dL) | 11 | 13.1 - 17.2 |
| MCV (FL) | 77 | 81 - 101 |
| MCH (PG/CELL) | 25 | 27 - 35 |
| RBC (× 106/UL) | 4.3 | 4.1 - 5.7 |
| WBC (× 103/UL) | 21.500 | 4.4 - 11 |
| Neutrophil (%) | 15700 (73) | 2500 - 7000 per microliter |
| Lymphocyte (%) | 5800 (27) | 1000 - 4500 per microliter |
| Platelet (UL) | 232.000 | 150.000 - 450.000 |
| BUN (Mg/DL) | 6 | 13 - 43 |
| Creatinine (Mg/DL) | 0.5 | 0.5 - 1 |
| Sodium (MEq/L) | 136 | (135 - 145) |
| Potassium (Mmol/L) | 3.7 | 3.6 - 5.2 |
| Calcium (Mg/DL) | 8.6 | 8.5 - 10.5 |
| Magnesium (Mg/DL) | 2.2 | 1.5 - 2.2 |
| Albumin (gr/DL) | 3.5 | 3.4 - 5.5 |
| PT (s) | 14 | 11 - 14 |
| PTT (s) | 42 | 25 - 35 |
| INR | 1.05 | 0.8 - 1.2 |
| ESR (Mmol)/h | 70 | 30 |
| C-reactive protein | 90 | 0 - 1⁺ |
| Blood glucose (Mg/dL) | 112 | 74 - 126 |
| Urine analysis | Normal | Normal |
| Blood culture | Staphylococcus aureus (coagulase. positive) | Negative |
| HIV-Ab | Non-reactive | Non-reactive |
| HBS-Ag | Non-reactive | Non-reactive |
| HCV-Ab | Non-reactive | Non-reactive |
Empirical antibiotic therapy was initiated with cefazolin (1 g intravenously every 8 hours) while awaiting definitive culture results. Supportive measures included intravenous (IV) fluid therapy and antipyretic treatment with acetaminophen (15 mg/kg every 6 hours as needed for fever). Clinical improvement was noted during the initial treatment course, with a significant reduction in erythema and swelling at the CIED pocket site. Upon confirmation of S. aureus from culture results, targeted antibiotic therapy was initiated.
The patient was scheduled for lead extraction. A temporary pacemaker was implanted via femoral vein access to maintain cardiac function during the procedure. The infected ventricular lead was successfully removed, but due to significant adhesions, the remaining two leads could not be extracted transvenously.
To ensure complete eradication of the infection, IV antibiotic therapy was continued for two weeks. Repeat blood cultures after this period were negative for bacterial growth. Subsequently, the patient underwent open-heart surgery to remove the remaining leads. During the procedure, a biological pulmonary valve replacement was performed due to clinical indications. Small lead fragments were intentionally left in the brachiocephalic vein to prevent significant vascular injury.
Postoperatively, the patient was closely monitored. Resolution of erythema and swelling was achieved, and no signs of infection were observed. The patient was discharged with a five-day course of oral cephalexin (500 mg four times daily) as prophylactic therapy, with instructions to report any signs of infection immediately. Follow-up evaluations were conducted monthly. At six months, the patient remained asymptomatic, with no recurrence of CIED infection, and all blood cultures remained negative throughout the follow-up period.
3.1. Conclusion and Follow-up
Following four days of IV antibiotic therapy, pacemaker analysis confirmed normal device function, and the patient’s fever and infection symptoms resolved. He was discharged on a five-day course of oral cephalexin (500 mg four times daily) as prophylactic therapy. The family was instructed to monitor for signs of recurrent infection, including fever, erythema, or swelling, and to report any concerns promptly. Scheduled follow-up evaluations included monthly clinical assessments and blood cultures at three-month intervals. At six months post-discharge, the patient remained asymptomatic, with no clinical or microbiological evidence of CIED infection. Blood cultures consistently returned negative results throughout the follow-up period. This case underscores the importance of timely intervention, strategic planning, and diligent follow-up in the effective management of pediatric CIED infections, contributing to favorable clinical outcomes.
4. Discussion
In this study, we reported a 6-year-old patient with recurrent infection of the CIED and this patient’s natural history. The implantation of CIEDs, including pacemakers and ICDs, has significantly increased over the past several years, primarily due to increased life expectancy and expanded indications from large clinical trials (12). This trend is especially notable in pediatric patients, where the implantation of ICDs has expanded in response to guidelines that have adapted adult data for younger populations. For pediatric patients with specific cardiovascular diagnoses, ICD implantation is generally recommended when a clear risk of sudden cardiac arrest is present, although there remain significant gaps in data guiding these recommendations (13).
Pediatric patients with CIEDs require regular follow-ups to monitor device function, manage complications, and adjust settings to accommodate their growth and development. This care involves a multidisciplinary approach, including cardiologists, electrophysiologists, and imaging specialists, to ensure optimal outcomes and minimize potential complications (14). Despite advancements in device technology and implantation techniques, complications related to CIEDs in pediatric patients remain a significant concern. These complications can include infection, lead dislodgement, and device malfunction. Infections, such as those seen in our 6-year-old patient, pose a serious risk and often require complex management strategies including antibiotic therapy and, in some cases, surgical intervention to remove and replace the device. These complications can significantly contribute to morbidity and mortality, highlighting the critical need for meticulous post-implantation care and prompt intervention when issues arise (15).
The incidence of CIED infection is estimated to be about 1.9 per 1000 devices per year, but according to the updated data from Baddour et al., the incidence rate is more specifically detailed in different contexts and settings, which might provide additional insights and updated figures (11). According to two recent prospective multicenter trials, the overall infection rate of CIEDs over 12 months is approximately 1%. De novo CIED implants carry a lower risk of infection compared to generator replacements, lead revisions, or upgrades. For instance, the infection rate at 12 months for new device implants ranged from 0.3% to 1.1%. In contrast, generator replacement procedures had an infection rate between 0.5% and 2.5%, while lead revision or upgrade procedures demonstrated an infection rate of 2.1% (16). The risk of in-hospital mortality in these patients is reported as high as 11.3% (17). In another study, this rate is estimated even higher and close to approximately 30% in an almost one-year follow-up (18).
There are known risk factors for the infection of CIEDs including renal failure, hematoma formation, implantation of multiple leads, and device revision (17). Infections commonly arise from the skin microbiota, often involving bacteria such as S. aureus, Staphylococcus epidermidis, and various Enterococci species. The main approach involves starting antibiotics, extracting the infected device, and reimplanting if necessary. However, there is a risk of reinfection, and subsequent extractions can be complicated (5). Interestingly, recent evidence suggests that extraction of the CIED may be omitted in some cases without increased risk of recurrent infection if there is no pocket infection or endocarditis (19).
Recent advances emphasize that therapeutic drug monitoring (TDM) and pharmacokinetic/pharmacodynamic (PK/PD) correlations during antibiotic therapy for CIED infections have been shown to improve clinical outcomes, particularly in reducing antibiotic resistance and healthcare costs. These approaches are increasingly being advocated for all patients with CIED infections to ensure optimal therapeutic levels and minimize adverse effects (20). In the Narui et al. study, 90.5% of patients had complete lead extraction using transvenous techniques. Repeat infection occurred in 9.5% of patients within a median of 103 days. The study identified LV assist devices, younger age, and S. aureus as risk factors, and CKD, CHF, septic emboli, S. aureus, and major complications as mortality predictors (18). Consistent with our study, repeated infections are more commonly seen in younger patients, with S. aureus infection and LV devices (21).
Many interventions have been proposed to reduce the risk of implant infection. The prophylactic antibiotic has shown a significant effect on declining this risk; however, other measures are yet to be proven as effective interventions (10). In a meta-analysis, no significant difference in mortality risk was found between men and women or between patients with PPM and ICD devices. While diabetes mellitus is a risk factor for infection, it does not significantly affect the mortality rate (21). Infection with the microorganism S. aureus is associated with a higher (20 - 30%) risk of mortality in these patients (22). The CIED infections can lead to complications like heart failure and emboli, which indicate a high probability of infective endocarditis and may necessitate open surgery (23). Identifying microorganism sensitivity (MSSA vs. MRSA) is crucial for antibiotic selection, but device extraction is the primary treatment for better outcomes. However, frail patients with severe comorbidities may not be able to undergo these procedures (24).
According to the 2021 PACES guidelines, lead extraction is recommended for CIED-related endocarditis, unexplained bacteremia (especially with S. aureus), or recurrent bacteremia unresponsive to antibiotics. Pre-extraction blood cultures and TTE are advised to guide antibiotic selection and assess embolic risks. For isolated superficial CIED pocket infections with negative blood cultures and no endocarditis, lead extraction may be considered (13). The overall risk of major complications in CIED removal is low (1.9%), but in-hospital mortality is relatively high (0.8%). Major complications include SVC perforation, laceration, and cardiac avulsion (25). In the LExICon study, major complications were 1.4% and mortality was 0.4%. Primary risk factors for complications and mortality during removal included low BMI, renal disease, heart failure, and extraction due to infection (26).
Preventive measures significantly reduce the risk of CIED infection and recurrent infections. These measures, detailed in Table 2, are divided into pre-intervention, peri-intervention, and post-intervention categories. Preprocedural antibiotics, such as IV cefazolin one hour before incision or IV vancomycin two hours before incision, are particularly effective in reducing implant infection risk (6).
| Measures | Process |
|---|---|
| Pre-procedural | For patients at higher risk, anticoagulation therapy can be maintained with either warfarin or non-vitamin K oral anticoagulants. According to the bruise control study, the target INR on the day of surgery should be ≤ 3.0 (or ≤ 3.5 for those with a mechanical valve); if this target is not met, the surgery is rescheduled. |
| If feasible, antiplatelet agents should be discontinued 5 to 10 days prior to CIED surgery. | |
| Chest hair should be removed using electric clippers, not razors, shortly before the surgery. | |
| Administer antimicrobial prophylaxis during the placement of a CIED. | |
| Peri-procedural | Surgical preparation should utilize 2% alcoholic chlorhexidine instead of povidone-iodine. |
| According to the WRAP-IT study, patients with a high risk of CIED infection should be considered for the use of an antibiotic envelope. | |
| Post-procedural | Hematoma drainage or evacuation should be avoided unless there is significant pain, tension, or wound dehiscence. |
| Postpone, or if possible, avoid any additional device re intervention or revision. |
Abbreviation: CIED, cardiac implantable electronic device.
a High-risk patients include those with atrial fibrillation and a CHA2DS2-VASc score of 4 or higher, a history of embolic events, or a mechanical valve.
4.1. Clinical Learning Point
The CIED infections, particularly in pediatric patients, pose significant risks and often necessitate intricate management strategies. Children with a prior history of pacemaker pocket infections are especially vulnerable, and meticulous screening for predisposing factors is essential. Despite the availability of strategies to mitigate the risk of recurrent infections, complete prevention at new implantation sites is not always guaranteed. Key preventive measures include minimizing the number of leads, effectively treating both local and systemic infections, conducting comprehensive immune evaluations, and utilizing a multidisciplinary team experienced in CIED procedures. This case report of a 6-year-old male with a history of TOF repair and recurrent pacemaker infections highlights the importance of prompt intervention, strategic planning, and diligent follow-up to mitigate the risks of infection, reduce mortality, and improve overall patient outcomes.