1. Background
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has significantly impacted global health, with varying effects across different population groups (1). While children generally experience milder symptoms compared to adults, certain subgroups, such as those with underlying morbidities, may be at higher risk for severe outcomes (2). Among these potentially vulnerable groups are children with malignancies, whose compromised immune systems, due to both their underlying condition and cancer treatments, may alter their response to SARS-CoV-2 infection (3). Although the peak of the pandemic has passed, understanding the impact of COVID-19 on pediatric cancer patients remains crucial. The potential long-term consequences of COVID-19 in this population are still under investigation. Additionally, new SARS-CoV-2 variants continue to emerge, potentially affecting vulnerable populations differently. Insights from this study may prove useful in developing strategies for managing future infectious disease outbreaks in pediatric oncology settings.
Recent studies have emphasized the need for a comprehensive understanding of how COVID-19 affects pediatric patients with malignancies compared to their counterparts without cancer. The clinical presentation of COVID-19 in children can range from asymptomatic to severe, with common symptoms including fever, cough, and gastrointestinal disturbances (4, 5). Laboratory findings often reveal alterations in WBC counts, inflammatory markers, and coagulation parameters (6, 7). Diagnostic tools such as reverse transcription-polymerase chain reaction (RT-PCR) and imaging techniques, particularly chest CT scans, have shown characteristic patterns that aid in diagnosing and monitoring disease progression (8). There is limited data on COVID-19 infection in children with cancer, and the interplay between malignancy and COVID-19 in pediatric patients remains an area of active research (9). Some studies suggest that children with cancer may experience more severe COVID-19 outcomes (10), while others report clinical courses similar to those of children without malignancies (11). Interestingly, at the beginning of the pandemic, it was speculated that patients with compromised immune systems might experience a milder clinical course (12).
2. Objectives
This study aimed to review the clinical symptoms, laboratory findings, and imaging results of COVID-19 in hospitalized children with malignancies, compared to their peers without malignancies, at Mofid Children's Hospital. The significant differences identified between these two groups could inform clinical decision-making and resource allocation in pediatric oncology units during the ongoing pandemic.
3. Methods
3.1. Study Design and Participants
We conducted a retrospective cohort study at Mofid Children's Hospital, a tertiary pediatric care center in Tehran, Iran, from July 2020 to December 2022. The study adhered to the principles of the Declaration of Helsinki and received approval from the Ethical Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RICH.REC.1400.026). The requirement for informed consent was waived due to the retrospective nature of the study.
Medical records of all pediatric patients admitted during this period who tested positive for SARS-CoV-2 by RT-PCR were reviewed. Inclusion criteria were: (1) Age ≤ 18 years, and (2) confirmed SARS-CoV-2 infection by RT-PCR. Exclusion criteria included outpatients who had positive PCR tests for SARS-CoV-2 infection.
Out of 1,313 eligible children, 105 had concurrent malignancies and were assigned to the case group. We then selected 105 age- and sex-matched children without malignancies as the control group using propensity score matching.
Propensity score matching is a statistical technique used to reduce confounding in observational studies by balancing covariates between groups (e.g., cases and controls). In this method, a propensity score is estimated for each individual, representing the probability of being assigned to a particular group (case or control) based on observed covariates. These scores are typically calculated using logistic regression. Once the propensity scores are estimated, individuals in the case group are matched to those in the control group based on similar propensity scores. This process creates pairs (or sets) of individuals with comparable characteristics across the groups, ensuring that any differences in outcomes between the groups are less likely to be due to confounding variables. By matching on the propensity score, the goal is to mimic the conditions of a randomized experiment, making the comparison between groups more valid (13).
3.2. Data Collection
We developed a data extraction form to collect the following information: Demographic data (age and sex), laboratory parameters [alanine aminotransferase (ALT) (14), prothrombin time (PT) (15), partial thromboplastin time (PTT), white blood cell count (WBC), hemoglobin (Hb), platelet count (Plt), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), and lung imaging findings chest X-ray and/or computed tomography (CT) (16) results]. The data were extracted from the hospital's electronic medical records system.
3.3. Statistical Analysis
We performed statistical analyses using SPSS software (version 26, IBM Corp., Armonk, NY, USA). The normality of continuous variables was assessed using the Shapiro-Wilk test. Descriptive statistics were presented as means and standard deviations for normally distributed continuous variables, medians and interquartile ranges for non-normally distributed continuous variables, and frequencies and percentages for categorical variables.
For between-group comparisons of patients with and without malignancy, we used the independent t-test for normally distributed continuous variables and the Mann-Whitney U test for non-normally distributed continuous variables. Categorical variables were compared using Pearson's chi-square test or Fisher's exact test, as appropriate. Logistic regression analysis was employed to evaluate the association between imaging findings and malignancy status, adjusting for potential confounders identified in univariate analysis. The relationship between log-transformed WBC and imaging findings was assessed using linear regression models.
We calculated odds ratios to compare mortality between the malignancy and non-malignancy groups. Pearson’s chi-square test was also used to examine the relationship between imaging findings and age groups. All statistical tests were two-tailed, and a P-value < 0.05 was considered statistically significant.
Given the sample size constraints of our study (210 children, 105 in each group), our ability to conduct comprehensive confounder analyses was limited. To address potential confounding, we used propensity score matching to control for age and sex, which were identified as the most critical potential confounders given our study design. While this approach helped balance these key variables between the case and control groups, we acknowledge that other potential confounders could not be fully accounted for in our analyses.
4. Results
4.1. Demographic and Clinical Characteristics
Our study included 210 children with confirmed SARS-CoV-2 infection: 105 with malignancies (case group) and 105 without malignancies (control group). The median age was 5 years (range: 0.08 - 17, IQR: 3 - 10.5), with 54.3% males and 45.7% females. In the malignancy group, acute lymphoblastic leukemia (ALL) was the most prevalent malignancy (n = 36, 34.3%), followed by brain tumors (n = 15, 14.3%). Acute myeloid leukemia (AML) and neuroblastoma were each observed in 11 children (10.5%). Other malignancies included Wilms' tumor (n = 10, 9.5%), non-Hodgkin’s lymphoma (n = 8, 7.6%), Hodgkin's lymphoma (n = 3, 2.9%), and chronic myeloid leukemia (CML) (n = 1, 0.95%). Additionally, a variety of rare tumors were identified (n = 6, 5.7%).
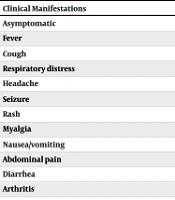
In this study, the clinical presentation differed significantly between the case and control groups (Table 1). Overall, 31.4% of all patients were asymptomatic.
| Clinical Manifestations | With Malignancy | Without Malignancy | P-Value |
|---|---|---|---|
| Asymptomatic | 21 (31.9) | 45 (68.1) | < 0.001 |
| Fever | 41 (42.3) | 56 (57.7) | 0.044 |
| Cough | 12 (42.9) | 16 (57.1) | 0.432 |
| Respiratory distress | 10 (32.3) | 21 (67.7) | 0.035 |
| Headache | 3 (42.9) | 4 (57.1) | 1.000 |
| Seizure | 2 (9.5) | 19 (90.5) | < 0.001 |
| Rash | 1 (20.0) | 4 (80.0) | 0.369 |
| Myalgia | 0 (0.0) | 1 (100.0) | 1.000 |
| Nausea/vomiting | 7 (23.3) | 23 (76.7) | 0.002 |
| Abdominal pain | 2 (25.0) | 6 (75.0) | 0.280 |
| Diarrhea | 8 (47.1) | 9 (52.9) | 0.816 |
| Arthritis | 0 (0.0) | 3 (100.0) | 0.246 |
a Values are expressed as No. (%).
The results showed a statistically significant association between malignancy and fever (P = 0.044), respiratory distress (P = 0.035), and nausea/vomiting (P = 0.002). Conversely, seizures were more frequent in patients with COVID-19 without malignancy (P < 0.001). Nine patients (4.3%) required intubation, with a higher number in the case group, although this difference was not statistically significant (P > 0.05).
4.2. Laboratory and Imaging Findings
Laboratory parameters revealed significant differences between the case and control groups (Table 2). White blood cell count count (P = 0.007), hemoglobin (P < 0.001), Plt count (P = 0.002), and ESR (P = 0.001) exhibited statistically significant variations between the malignant and non-malignant groups. Among these parameters, ESR demonstrated the most clinical relevance.
Among the 114 patients (54.29%) who underwent chest imaging, ground-glass opacity (GGO) was significantly associated with malignancy (P = 0.003, Table 3).
| Laboratory Parameters | With Malignancy | Without Malignancy | P-Value |
|---|---|---|---|
| WBC | 7147 ± 8276.69 | 8382 ± 5593.78 | 0.007 |
| Hb | 10.2 ± 2.24 | 11.6 ± 1.96 | < 0.001 |
| Plt | 212984 ± 173.83 | 266839 ± 134884 | 0.002 |
| ESR | 40 ± 28.3 | 28 ± 22.6 | 0.001 |
| CRP | 21.86 ± 20.3 | 17.6 ± 17.5 | 0.097 |
| LDH | 781 ± 524.4 | 707 ± 545 | 0.174 |
| AST | 51 ± 46.4 | 86 ± 309.99 | 0.856 |
| ALT | 43 ± 41.1 | 65 ± 252.9 | 0.033 |
| PT | 13 ± 6.6 | 14 ± 12.2 | 0.818 |
| PTT | 33 ± 14.46 | 32 ± 8.3 | 0.778 |
Abbreviatons: ALT, alanine aminotransferase; Plt, platelet; PT, prothrombin time; PTT, partial thromboplastin time; WBC, white blood cell count; Hb, hemoglobin; ESR, erythrocyte sedimentation rate; CRP, C-reactive.
a Values are expressed as mean ± SD.
| COVID Patients | Diffuse Infiltration | GGO | Consolidation | Perihilar Haziness | Emphysema | No Imaging Performed |
|---|---|---|---|---|---|---|
| With malignancy | 7 | 9 | 8 | 9 | 1 | 56 |
| Without malignancy | 10 | 22 | 5 | 7 | 0 | 40 |
| P-value | 0.302 | 0.003 | 0.521 | 0.785 | 1.000 | 0.108 |
| Odds ratio (95% CI) | 0.095 (0.009, 1.071) | 0.050 (0.005, 0.559) | 0.346 (0.030, 3.982) | 0.227 (0.022, 2.367) | 2.69 (0.11, 67.3) | 1.662 (0.893, 3.094) |
4.3. Complications and Mortality
Thrombotic events were very rare, with only two cases observed in the initial cohort of 1,313 children: One case of deep vein thrombosis (DVT) in a child with a brain tumor and one case of cerebral sinus venous thrombosis (CSVT) in a child without an underlying disease.
No statistically significant association was found between mortality and malignancy status (P = 0.105, Table 4).
| COVID Patients | Mortality in COVID Patients | Odds Ratio (95% CI) | P-Value | |
|---|---|---|---|---|
| Yes | No | |||
| With malignancy | 10 | 94 | 2.686 (0.815, 8.857) | 0.105 |
| Without malignancy | 4 | 101 | ||
5. Discussion
The COVID-19 pandemic posed significant challenges for pediatric oncology patients, raising concerns about the vulnerability and outcomes of children with malignancies infected with SARS-CoV-2. Our study provided detailed data on the clinical presentation, laboratory findings, and imaging characteristics of COVID-19 in children with and without malignancies.
In our study, 1,313 eligible children were identified, of whom 105 with concurrent malignancies were included as cases. These cases were matched by age and sex with 105 controls without malignancies. While the matched design enhances comparability between the groups, the limited number of cases may reduce the power to detect smaller associations or interactions, which should be taken into account when interpreting the findings.
The demographic characteristics of our study population, including the age distribution and gender ratio, were consistent with those reported in several other studies on pediatric COVID-19 cases (17, 18). Among the malignancy group in our study, ALL was the most prevalent, followed by brain tumors and AML, which aligns with global data on pediatric cancer distributions during the pandemic (19, 20).
The present study revealed significant differences in clinical presentation between children with and without malignancies. The higher prevalence of fever, respiratory distress, and gastrointestinal symptoms in the malignancy group was noteworthy and aligns with previous studies suggesting that immunocompromised patients may experience more severe symptoms during COVID-19 infection (10). In a systematic review of multisystem inflammatory syndrome (MIS-C) involving 7,297 children, the most common symptoms were fever (98.7%), rash (55.2%), and conjunctivitis (52.8%) (21).
A systematic review of 45 studies involving pediatric cancer patients across five continents highlighted that ALL was the most commonly reported malignancy, followed by AML and other solid tumors such as Wilms’ tumor and neuroblastoma. The review also found that while the majority of infections were mild or moderate in severity, a significant subset of patients required hospitalization, with a notable incidence of severe COVID-19 cases (20).
The laboratory findings in our study provided valuable insights into potential differences in immune response between the two groups. Significant variations in WBC count, hemoglobin, Plt count, and ESR between the malignant and control groups suggest that the underlying malignancy may influence the hematological response to SARS-CoV-2 infection. A study by Tolunay et al., which reviewed medical records of pediatric patients with blood malignancies hospitalized due to COVID-19 at Children's Hospital Los Angeles, found that while most patients exhibited mild to moderate symptoms, significant hematologic abnormalities, including changes in WBC and Plt counts, were prevalent. These findings reflect the complex interplay between cancer treatment and COVID-19 infection (22).
These results underscore the need for careful monitoring of these parameters in pediatric cancer patients with COVID-19.
Imaging findings in the present study provided significant insights contributing to the literature on COVID-19 in pediatric patients, particularly those with malignancies. Ground-glass opacity (GGO) was significantly more associated with malignancy, suggesting that children with malignancies may experience more severe lung involvement. A comprehensive systematic review by Shelmerdine et al. identified GGO as a common finding in pediatric COVID-19 cases (23). The increased observation of GGO in children with malignancies in the present study aligns with their findings, suggesting that underlying health conditions can influence imaging results. Additionally, we found a relationship between diffuse infiltration on chest imaging and increased WBC count, which may be explained by the occurrence of systemic inflammatory responses in pediatric COVID-19 patients.
While our study found no statistically significant difference in mortality between the malignancy and non-malignancy groups, the slightly higher odds of death in children with malignancies warrant careful consideration. This finding contrasts with some early pandemic hypotheses suggesting that immunosuppressed patients might experience a milder clinical course (12). However, it aligns with more recent studies indicating potentially worse outcomes for children with cancer who contract COVID-19 (15).
Recent global studies have provided further insights into COVID-19 outcomes in pediatric cancer patients. A global registry study by Mukkada et al. reported that while most pediatric cancer patients with COVID-19 experienced mild symptoms, a significant minority required hospitalization or intensive care support (24). Conversely, a study by Kebudi et al. found similar clinical presentations between pediatric cancer patients and the general pediatric population, contrasting with our observation of more severe symptoms in the malignancy group (25). These differences underscore the need for region-specific data to guide local clinical practices.
The very low incidence of thrombotic events in our cohort was encouraging, given the established concerns about COVID-19-associated coagulopathy.
While our study focused on immediate outcomes, the potential long-term effects of COVID-19 in pediatric cancer survivors warrant further investigation. Emerging evidence indicates that some COVID-19 survivors, including children, may experience persistent symptoms, commonly referred to as "long COVID." For pediatric cancer patients, who already contend with long-term health challenges, the additional burden of this condition could have significant implications.
The findings of this study hold important implications for future pandemic preparedness in pediatric oncology settings. For instance, the higher prevalence of respiratory distress and ground-glass opacities in children with malignancies suggests that these patients may benefit from earlier and more aggressive respiratory support. As healthcare systems worldwide continue to refine their pandemic response plans, incorporating these insights can help optimize care for this vulnerable population during future outbreaks.
5.1. Limitations
Our study had several limitations that should be acknowledged. Its retrospective, single-center design may introduce potential biases and limit the generalizability of the findings. The relatively small sample size (210 children) constrained our ability to perform comprehensive confounder analyses and may have limited the generalizability of the results, particularly in subgroup analyses. While we controlled for age and sex through propensity score matching, other potential confounders, such as specific cancer types, treatment status, and comorbidities, could not be fully accounted for in our analyses. This limitation may have impacted our ability to detect more subtle differences between groups or to fully assess the independent effect of malignancy on COVID-19 outcomes.
The study period, spanning from July 2020 to December 2022, encompassed a time during which COVID-19 management strategies evolved, potentially influencing patient outcomes. We were also unable to account for the effects of specific cancer treatments or disease stages on COVID-19 outcomes. Furthermore, as our study predates the widespread availability of COVID-19 vaccines, we could not assess the impact of vaccination in this population.
5.2. Conclusions
In conclusion, our findings suggest that children with malignancies who contract COVID-19 may present with more severe symptoms and distinct laboratory and imaging findings compared to their counterparts without malignancies. Although mortality rates were not significantly different, the higher odds of death in the malignancy group highlight the need for heightened vigilance and potentially more aggressive management strategies for this vulnerable population.
Future research should prioritize multi-center prospective studies with larger sample sizes to further elucidate the long-term outcomes and potential complications of COVID-19 in pediatric cancer patients and to more robustly assess the impact of potential confounders on these outcomes.