1. Introduction
Acute liver failure (ALF) is a rare, potentially fatal condition characterized by rapid deterioration of liver function, which can lead to significant morbidity and mortality if not managed properly. The causes of pediatric acute liver failure (PALF) are various, including viral infections, metabolic disorders, autoimmune diseases, and drug-induced liver injury (1). Viral infections are a recognized cause of ALF, with common etiologies including Hepatitis A, B, C, and E, as well as various members of the Herpesviridae family like herpes simplex virus (HSV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV), along with the enteroviruses, Adenovirus, and coronavirus-2 (SARS-CoV-2) (1). In rare instances, human herpesvirus 6 (HHV-6), another Herpesviridae member, has also been associated with PALF. In recent years, particularly following the 2022 hepatitis epidemic, which revealed a concerning association between HHV-6 and severe liver complications (2), there has been growing evidence linking HHV-6 infection to various clinical syndromes, including hepatitis and ALF.
This report presents a pediatric case of ALF attributed to HHV-6 infection, aiming to enhance awareness among clinicians about this rare association. It contributes to the existing literature by highlighting a unique instance where HHV-6 was identified as the sole potential cause of ALF after extensive testing. This underscores the need to include HHV-6 in viral screening protocols for liver failure of unknown etiology and highlights the importance of further research to establish more definitive diagnostic criteria and causal links between HHV-6 and ALF.
2. Case Presentation
We present the case of a 7-month-old girl with a two-day history of fever, diarrhea, and agitation. The patient's prior medical history was unremarkable. Neither the patient nor her family had traveled to epidemic zones, nor were they exposed to toxins or contaminated water or food. The patient had a low-grade fever, brown stool, and stable vital signs. Initial evaluations revealed a WBC of 13,400 cells/mm3, hemoglobin within normal limits, and an elevated C-reactive protein level of 2 mg/L, as shown in Table 1.
| Parameters | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| WBC, cells/mm3 | 13,400 | 31,000 | - |
| Neutrophils, % | 55 | 35 | - |
| Lymphocytes, % | 40 | 57 | - |
| Hemoglobin, g/dL | 11.5 | 10.5 | - |
| CRP, mg/L | 2 | - | |
| Na, mmol/L | 136 | 133 | - |
| K, mmol/L | 4.1 | 5 | - |
| BUN, mmol/L | 2 | 28 | - |
| Cr, mg/dL | 0.4 | 0.43 | - |
| ALT, U/L | - | - | 1141 |
| AST, U/L | - | - | 4140 |
| ALP, U/L | - | - | 486 |
| PTT, s | - | - | 77 |
| INR | - | - | 1.51 |
| AFP, ng/mL | - | - | 111 |
| Bilirubin, mg/dL | - | - | Total: 0.8, direct: 0.3 |
| LDH, mg/dL | - | - | 561 |
| Alb, U/L | - | - | 3.3 |
| Ca, g/dL | - | - | 8.2 |
| Mg, mg/dL | - | - | 2.7 |
| Ammonium, mg/dL | - | - | 113 |
| Lactate, micromole/L | - | - | 11.4 |
| Troponin, mmol/L | - | - | 14.5 |
| ANA, ng/mL | - | - | < 1:80 |
| SMA | - | - | < 1:20 |
| Ceruloplasmin | - | - | 32 |
| Copper, mg/dL | - | - | 95 |
| ABG, µg/dL | - | - | pH: 7.38, PaCO2: 46 mmHg, PaO2: 78 mmHg, HCO3⁻: 25 mEq/L, base excess: -1 mEq/L |
Abbreviations: AFP, alpha-fetoprotein; Alb, albumin.
Following the standard approach to gastroenteritis, we initiated hydration therapy and ordered stool examination studies. The fever subsided by the second day, and the diarrhea improved, with stool examination results returning unremarkable. However, the patient remained agitated. Abdominal ultrasonography was performed due to suspicion of intussusception but revealed no signs, with normal findings in the liver and other tissues. On the third day, the patient developed abdominal distention and palpable hepatomegaly. Laboratory testing revealed markedly elevated liver enzyme levels, with ALT levels at 1141 U/L, AST levels at 4140 U/L, and ALP levels at 486 U/L. As shown in Table 1, PT, PTT, and INR were elevated, while Alpha-fetoprotein levels were mildly elevated, and bilirubin levels were within normal limits. Fresh frozen plasma and vitamin K were administered, but the INR values were not corrected. Based on the lack of any known history of liver disease and evidence of acute liver injury, the uncorrected coagulopathy despite vitamin K administration, and the patient's agitation as a sign of encephalopathy, the diagnosis of PALF was made. Subsequent abdominal ultrasound revealed hepatomegaly with a heterogeneous appearance and a thickened gallbladder wall. A comprehensive workup was performed to evaluate the underlying etiology of PALF.
Drug-induced liver injury was ruled out as there was no history of exposure to hepatotoxic drugs or herbal medicines, and acetaminophen was administered at a low therapeutic dose (10 mg/kg q6h) prior to the onset of symptoms. Toxicology screening returned negative results for acetaminophen, salicylates, ethanol, illicit substances such as cocaine, opioids, methadone, amphetamines, marijuana and their metabolites, benzodiazepines, barbiturates, heavy metals (including lead, arsenic, and mercury), as well as antiepileptic drugs, including phenytoin, carbamazepine, lamotrigine, and valproate. Autoimmune hepatitis was considered but was ruled out due to negative autoantibody tests (ANA and SMA) (Table 1) and a clinical presentation inconsistent with autoimmune liver disease. Hemophagocytic lymphohistiocytosis was considered due to the patient’s fever, diarrhea, hepatomegaly, and encephalopathy. However, the absence of key diagnostic features, such as significant splenomegaly, cytopenia, markedly elevated inflammatory markers, or other systemic manifestations, ruled it out as a cause.
Metabolic and genetic conditions were deemed unlikely given the patient’s normal baseline health, absence of relevant family history, and clinical presentation. Targeted testing for specific conditions was performed. Wilson disease was excluded due to normal serum ceruloplasmin and copper levels (Table 1). Tyrosinemia type 1 was ruled out based on negative newborn screenings and the absence of characteristic clinical signs such as sepsis. Galactosemia was excluded due to the lack of typical clinical signs, including failure to thrive, jaundice, or sepsis. Urea cycle defects were initially considered due to elevated ammonia levels (113 µmol/L) and associated symptoms of diarrhea and encephalopathy. However, typical ammonia levels in Urea cycle defects are significantly higher, and the lack of hypoglycemia or metabolic acidosis (Table 1) further supported their exclusion. Mitochondrial dysfunction was considered given the elevated lactate level (11.4 mmol/L). However, the lack of multisystem involvement or developmental delay made mitochondrial hepatopathy an unlikely cause. Rare genetic disorders, such as NBAS deficiency or Niemann-pick type C, were also ruled out given the absence of syndromic features or recurrent liver failure episodes. Hereditary fructose intolerance was excluded due to no exposure to fructose-containing foods and no relevant clinical features.
Cardiovascular causes were ruled out following a normal cardiac examination and echocardiography, with no evidence of hypoperfusion, Budd-Chiari syndrome, or veno-occlusive disease. Hematological malignancies were excluded based on peripheral blood smear findings and the absence of systemic signs such as significant splenomegaly or lymphadenopathy. Lastly, a thorough evaluation for viral etiologies was conducted using multiplex PCR testing, which included SARS-CoV-2, influenza A and B, Hepatitis viruses A - E, adenovirus, EBV, parvovirus B19, CMV, HSV, HHV-6, and 7.
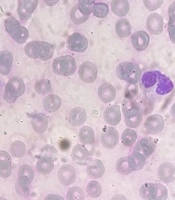
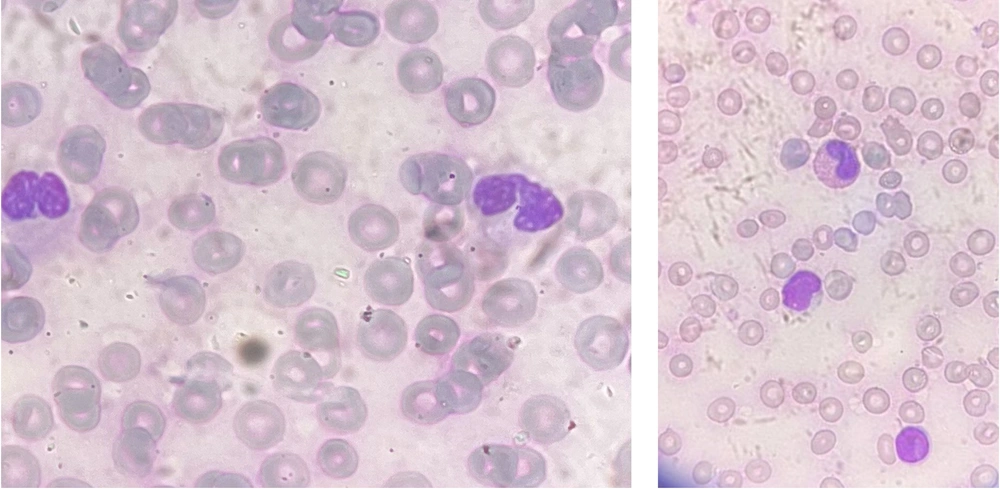
Eight hours later, the patient had a tonic-clonic seizure, which was managed with diazepam. At this point, lymphocytic leukocytosis, decreased hemoglobin, thrombocytopenia, hypocalcemia, high lactate dehydrogenase, elevated ammonia levels, and negative blood culture were present, as shown in Table 1. The peripheral blood smear, as shown in Figure 1, contained large atypical monocytes (5%), a high neutrophil-to-lymphocyte ratio, lobulated neutrophils, and Pseudo-Pelger-Huet cells (approximately 20 - 30% of all neutrophils).
Pseudo-Pelger-Huet cells are hypopigmented, hypogranular neutrophils with bilobed, dumbbell-shaped nuclei and coarse clumps of nuclear chromatin. Pseudo-Pelger-Huet anomaly is an acquired condition that can be caused by various factors, including neoplastic, nutritional, autoimmune, drugs and toxins, and infections, such as HIV, parvovirus B19, HCV, and HHV-6 (3). Based on the test results, the patient was managed for hepatic encephalopathy and was considered a liver transplant candidate. In addition to dietary adjustments, lactulose was prescribed for hepatic encephalopathy. Her ammonia levels, liver function tests, and mental status were all monitored regularly.
On the fourth day of hospitalization, the patient developed respiratory distress, and her oxygen saturation decreased. A chest X-ray showed bilateral pleural effusion, necessitating chest tube insertion and intubation. Pleural fluid analysis indicated a transudative nature. Despite aggressive interventions, the patient succumbed to the illness. The result of the multiplex PCR test was returned after the patient's death, showing that tests for hepatitis A-E, adenovirus, EBV, CMV, parvovirus B19, HSV, HHV-7, SARS-CoV-2, and influenza A and B were all negative. However, HHV-6 was detected in the viral evaluation, with a cycle quantification (CQ) value of 26. While the exact viral load was not determined, the CQ value of 26 is consistent with a clinically significant level of HHV-6 DNA. While it does not necessarily indicate the causation of HHV-6, it was the only possible etiology contributing to the development of ALF and her death.
Viral genomes were extracted using HighPure Viral Nucleic Acid Kits (Roche Diagnostics GmbH). Real-time PCR assays were conducted using Hanagene kits (Arak, Iran), which are validated for diagnostic use. To ensure reliability, the positive result for HHV-6 was confirmed with an independent test using the HHV6 Real-TM Quant kit (Sacace, Italy).
After the patient’s death, an autopsy was performed, which confirmed the cause of death as ALF. During the autopsy, a liver biopsy was obtained, and HHV-6 was detected in the hepatic tissue, further supporting the role of HHV-6 as the causative agent. One significant limitation of this study is that a liver biopsy and detection of HHV-6 were not performed before the patient's death. Earlier tissue-based testing would have allowed for a more definitive assessment of the virus's role in the progression of ALF and would have provided the opportunity to calculate the viral load in the tissue. Patient privacy and data security were rigorously maintained by anonymizing all identifying information, securely handling data, obtaining informed consent from the parents, and adhering to ethical guidelines reviewed by the relevant institutional review board.
3. Discussion
Acute liver failure is a rare, acute, and possibly reversible condition characterized by abrupt and severe hepatocellular injury resulting in severe hepatic impairment and rapid clinical deterioration in patients without prior liver disease (4). Pediatric acute liver failure is defined as (1): Acute onset of liver disease with biochemical evidence of acute liver injury without evidence of chronic liver disease along with coagulopathy uncorrectable by vitamin K in either of the following forms:
(1) PT > 15s or INR > 1.5 with encephalopathy
(2) PT > 20s or INR > 2 with or without encephalopathy
In our case, elevated levels of ALT, AST, PT, PTT, INR, LDH, and ammonia, along with an abdominal ultrasound showing the transition from a normal liver to hepatomegaly with a heterogeneous appearance, indicated acute liver injury. The presence of agitation, a sign of encephalopathy, along with an INR exceeding 1.5 despite vitamin K administration, confirmed the diagnosis of PALF.
Acute liver failure can manifest as a complex multi-organ system process, leading to cerebral edema, respiratory failure, renal failure, hemodynamic instability, coagulopathy, and sepsis. The initial presentation of PALF is generally mild, often beginning with prodromal viral-like illness days to weeks prior to the onset of more severe symptoms (5), during which patients may experience nonspecific signs such as fever, nausea, vomiting, loss of appetite, fatigue, malaise, jaundice, pruritus, and abdominal discomfort. As the condition progresses, clinical manifestations of ALF may develop, including icterus, hepatomegaly, bruising/bleeding, seizures, and encephalopathy (6).
In the case of our patient, the initial clinical manifestations upon admission were diarrhea, fever, and agitation. Subsequently, the patient developed abdominal distension, hepatomegaly, and seizures. The underlying etiology of PALF can be broadly classified as immunologic, toxin- or drug-related liver injuries, metabolic, infectious, cardiovascular, and oncologic. These include autoimmune hepatitis, hemophagocytic lymphohistiocytosis, acetaminophen toxicity, non-APAP medications (such as herbals and dietary supplements, analgesics, antimicrobials, antiepileptics, and recreational drugs), metabolic and genetic diseases like Wilson disease, tyrosinemia, galactosemia, urea cycle defects, fatty acid oxidation disorders, mitochondrial dysfunction, and hereditary fructose intolerance. Viral causes include hepatitis A-E, HSV, EBV, CMV, adenovirus, enteroviruses, and SARS-CoV-2. Additionally, ischemic liver injury secondary to Budd-Chiari syndrome, veno-occlusive disease, cardiac dysfunction, and hematological malignancies such as leukemia and lymphoma can also contribute (1).
The cause of over 50 percent of PALF cases is not determined, leading to the classification of liver failure of unknown etiology (LFUE) (5). Although it is assumed that a virus may play a role in LFUE, the exact virus or viruses responsible remain unknown. Our patient had no history of using hepatotoxic drugs or herbal supplements. Acetaminophen was administered at a minimal therapeutic dose of 10 mg/kg, and toxicology results were negative. There was no clinical or laboratory evidence suggesting autoimmune hepatitis, hemophagocytic lymphohistiocytosis, or any metabolic or genetic disorders related to ALF. Hematological malignancies and cardiovascular issues were also excluded. After ruling out common viral causes of ALF, the patient's condition was determined to be either LFUE or attributed to HHV-6, the only viral pathogen detected.
The mortality rate among LFUE patients is notably high. Due to the unknown underlying cause, LFUE responds poorly to medical treatments and often necessitates liver transplantation. The transplant-free survival rate in LFUE cases is less than 25%, and even following transplantation, outcomes are less favorable compared to many other transplantation indications (7). In developed countries, viral causes of PALF are uncommon. In contrast, infectious diseases, such as hepatitis viruses A-E, are the leading cause of ALF in developing countries. While HHV-6 typically presents as a self-limited infection associated with exanthem subitum, its frequent identification in liver biopsies of patients with ALF suggests a potential link. HHV-6 is usually acquired early in life, between six months and two years, after maternal antibodies decline and commonly causes asymptomatic or mild infections. Primary HHV-6 infections often present as acute febrile illnesses in children, with symptoms such as fever, skin rash, and gastrointestinal and respiratory tract symptoms. The hallmark manifestation is exanthema subitum. However, in rare cases, primary HHV-6 infection can lead to more severe diseases such as thrombocytopenia, infectious-mononucleosis-like syndrome, gastroenteritis, myocarditis, neurological complications, the development of malignancies through immune modulation, and hepatitis, including fulminant forms and ALF (8, 9).
HHV-6 was initially linked to LFUE in 1990 when it was identified in an infant who succumbed to LFUE. Subsequent investigations have continuously shown that HHV-6 is more commonly identified in liver biopsies of children with LFUE compared to the control group. According to a recent study, pediatric liver transplant recipients due to LFUE had a higher prevalence and greater quantity of HHV-6 in their liver tissue compared to the control group (7). The end of COVID lockdowns has led to an increased prevalence of common pediatric infections and systemic complications. Previously shielded children suddenly exposed to diverse pathogens might have triggered an atypical immunological response. HHV-6 is one of these common pathogens and its identification and possible role in the recent acute hepatitis outbreak as the third most frequently identified pathogen, following adenovirus and SARS-CoV-2, in recent UK cases of acute non-A-to-E hepatitis, highlights its potential to cause severe disease, particularly in previously uninfected children (2).
The relationship between HHV-6 infection and ALF is complex and multifaceted. Evidence linking HHV-6 to liver injury typically hinges on two factors: (1) The detection of the virus in the blood or liver tissue; and (2) the exclusion of other etiologies of ALF. Detectable viremia is a hallmark of active infection; however, it alone does not establish a causal link between HHV-6 and LFUE, nor does it differentiate between primary infection and reactivation (7). In cases of ALF with detectable HHV-6 viremia, this finding may reflect two distinct scenarios. It could indicate a primary HHV-6 infection directly causing ALF, or it might represent HHV-6 reactivation secondary to liver failure caused by another etiology, rather than HHV-6 being the primary cause of ALF itself. Reactivation of HHV-6 from latency occurs frequently in patients with severe immunosuppression and occasionally in immunocompetent patients. In liver transplant recipients, HHV-6 is increasingly recognized as a pathogen capable of causing primary infection or reactivating from latency, leading to a range of adverse clinical syndromes such as fever, hepatitis, and encephalitis (10).
While HHV-6 has been linked to liver dysfunction, most studies attribute its role to reactivation rather than direct causation. A recent study in which HHV-6 was found in a significant number of pediatric cases of LFUE suggested that reactivation of this virus may have contributed to the clinical picture rather than being the primary cause. While the relevance of HHV-6 was not entirely denied, HHV-6 was suggested as a helper virus to adeno-associated virus 2 (11). Therefore, HHV-6 detection has often been assumed to be coincidental, and its role as the main cause of ALF has been ignored.
Thus, while there is growing evidence linking HHV-6 to ALF, its precise role remains elusive. Ongoing efforts aim to establish more definitive frameworks for this link. For instance, a recent study identified a viral load cut-off of 23,357 copies/106 cells in liver tissue samples as a threshold for attributing cases of LFUE to HHV-6, with a sensitivity of 0.853 and specificity of 0.579. For children under six, the optimal cut-off was 73,723 copies/106 cells, while a sensitivity of 1.0 was achieved at 7.3 × 103 copies/106 cells across all age groups (7). Additionally, histopathological evaluation may help distinguish HHV-6-related ALF from other causes of hepatitis. Recent studies suggest that a centrilobular pattern of necroinflammation characterized by central perivenulitis and confluent centrilobular to panlobular necrosis, along with hepatocellular swelling and portal inflammation in liver tissue, may be a key predictor of HHV-6-related acute severe hepatitis (12).
As HHV-6 has a high seroprevalence (72% - 95%), most institutions rarely incorporate HHV-6 testing in their standard LFUE workup (7). However, considering that the etiology of ALF remains unidentified in 40% to 50% of patients and the fact that clinical outcomes are significantly influenced by the underlying cause, with viral and unknown etiologies resulting in poorer outcomes, the absence of comprehensive viral screening in clinical practice seems illogical.
Roseolovirus infection typically does not need specific treatment, and aside from encephalitis, there is insufficient evidence to recommend treatment for other HHV-6-associated end-organ diseases. Despite the lack of controlled studies, foscarnet and ganciclovir are recommended as the first line of treatment, followed by cidofovir as the second. High dosages of foscarnet at 60 mg/kg twice a day or ganciclovir at 18 mg/kg/day are suggested for HHV-6-associated CNS infections (13). Although given the transient nature of most HHV-6 infections, as well as the significant risk of side effects associated with antivirals, such as myelosuppression with ganciclovir and nephrotoxicity with foscarnet and cidofovir, treatment decisions must be made based on the specific conditions of each case (14).
In conclusion, the complexity of ALF highlights the importance of thorough investigation, particularly in cases where the etiology remains unidentified. This case report highlights the crucial role of HHV-6 as a potential cause of ALF in a pediatric patient, underscoring the need for clinicians to consider this virus when faced with LFUE. Our findings reveal that HHV-6 was the only identifiable cause of ALF after extensive testing for other common causes, marking a significant contribution to understanding this rare association. This case emphasizes the importance of including HHV-6 in viral screening protocols for pediatric LFUE, especially when other causes have been ruled out. Given the undetermined etiology of over 40% of cases of PALF and the high mortality rates among them, considering uncommon viral etiologies such as HHV-6 is imperative. Lastly, we strongly encourage further research to understand the role of HHV-6 in ALF, investigate the mechanisms by which HHV-6 contributes to liver failure, evaluate treatment options, and develop targeted therapeutic strategies to improve patient outcomes.