1. Background
Coronaviruses (CoVs) are ribonucleic acid (RNA) viruses that infect a wide range of host species, including domestic and farm animals as well as bats. They were first identified in the 1960s (1). This viral family can cause a variety of diseases, ranging from the common cold to severe acute respiratory syndrome (SARS) in humans (2). Common symptoms include fever, cough, chest pain, particularly tightness, and dyspnea or shortness of breath. Many cases experience a mild course of the disease (3). Studies have shown that pregnant women with SARS have worse outcomes compared to non-pregnant women of the same age (4). Spontaneous abortion has been reported in cases infected with SARS during the first trimester. Additionally, intrauterine growth restriction (IUGR) and preterm birth have been observed in pregnancies affected by SARS in the second and third trimesters. Neonatal intensive care unit (NICU) admission, endotracheal intubation, renal failure, and maternal mortality have also been noted in this population (5). However, no cases of vertical transmission of SARS-CoV from mother to fetus have been confirmed (6).
Study by Yu et al. (7) and Schmid et al. (8) found no perinatal SARS infections in neonates born to mothers infected during pregnancy. Khan et al. suggested that COVID-19 might result in long-term congenital disorders in neonates through infections, which is particularly concerning in resource-constrained developing countries where prenatal screening is often lacking. The prevalence of COVID-19 in these countries remains high (9).
Pregnancy is typically managed by obstetricians and gynecologists, while COVID-19 is handled by virologists and infectious disease specialists. Therefore, intersectoral collaboration and information exchange are crucial to prevent complications in neonates (9). Notably, some pregnancies have had good outcomes despite maternal SARS infection (10). Given that 68 - 85% of SARS-CoV-2 cases share sequence similarity with SARS, COVID-19 may follow a similar trend in pregnant women. Although SARS-CoV-2 shares similarities with other CoVs, it appears to be more contagious across different populations (6). Consequently, pregnant women and neonates should be prioritized in COVID-19 prevention and management strategies (11).
In a previous study, we evaluated maternal and neonatal outcomes in pregnancies positive for SARS-CoV-2 over a 6-month period (2019 - 2020). This retrospective study included 100 SARS-CoV-2-positive pregnant women and 50 healthy controls from hospitals affiliated with Zahedan University of Medical Sciences across five cities in Sistan and Baluchestan. The results indicated an increased risk of preterm birth and associated complications, as well as varied maternal and neonatal outcomes among SARS-CoV-2-positive pregnancies (12).
2. Objectives
Building on these findings, the current study was designed to evaluate additional characteristics and parameters with a more comprehensive approach. Conducted over a one-year period (September 2020 to September 2021) in Zahedan, this study aimed to provide a deeper understanding of the impact of COVID-19 on maternal and neonatal health in our province by analyzing a broader set of data and outcomes. Therefore, the present study aimed to evaluate the impact of COVID-19 on maternal and neonatal outcomes by comparing a group of COVID-19-positive pregnant mothers with a control group of healthy pregnant mothers in Zahedan, Sistan and Baluchestan province, Iran.
3. Methods
This retrospective study employed a case-control research design. The statistical population consisted of case and control groups, including the medical records of all pregnant women admitted to the maternity ward of Ali-Ibn-Abi-Taleb Hospital, Zahedan, Iran, from September 2020 to September 2021. The study commenced upon receiving approval from the Research Council of the School of Medicine at Zahedan University of Medical Sciences, Zahedan, Iran (IR.ZAUMS.REC.1401.013).
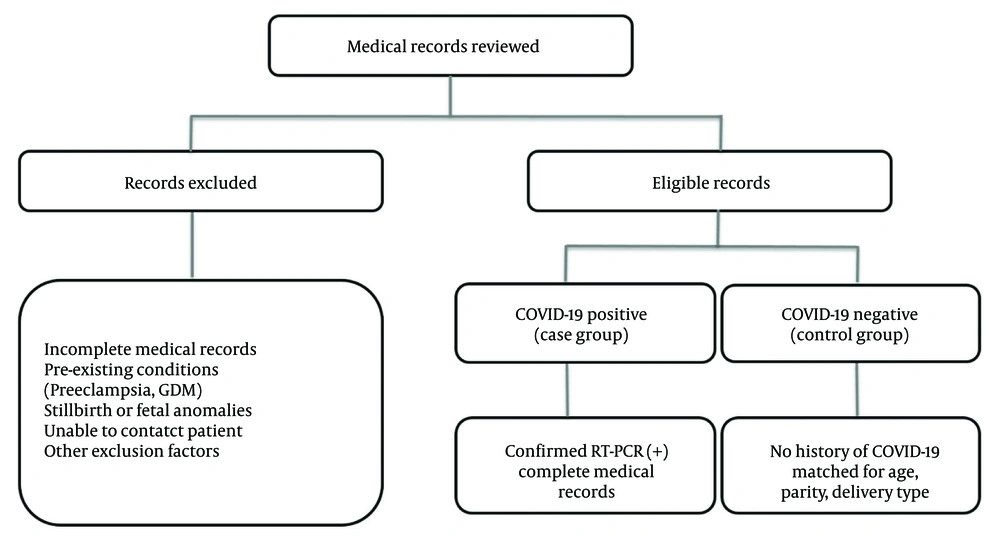
The inclusion criteria for the case group comprised the medical records of all pregnant women admitted to the maternity ward with a documented history of COVID-19 infection during pregnancy, confirmed through a positive RT-PCR test, provided that these records were complete. Exclusion criteria included a history of preeclampsia, gestational diabetes mellitus (GDM), stillbirth, fetal anomalies, incomplete medical records, and the inability to contact the pregnant women when records were deficient.
Once included in the study, relevant data such as maternal age, neonatal gender, parity, type of delivery, 1- and 5-minute Apgar scores, gestational age, timing of maternal COVID-19 infection, neonatal mortality up to 30 days, neonatal admission to the NICU, and low birth weight (LBW) were extracted from the medical records and entered into the information form. Missing data were carefully addressed. For any missing data from the medical records, efforts were made to contact the mothers directly to obtain the required information. If this was not possible, the data were considered missing and excluded from the respective analysis. The sample selection process, including inclusion and exclusion criteria, is illustrated in Figure 1.
The study sample comprised all available and eligible cases within the study period, totaling 180 pregnant women, divided into case (n = 90) and control (n = 90) groups. The case group included all pregnant women with confirmed COVID-19 infection during the study period who met the inclusion criteria. The control group was selected by matching non-infected pregnant women based on maternal age, type of delivery, and parity to ensure comparability. As this was a retrospective study, the sample size was not determined by an a priori power calculation but rather reflected the total number of eligible cases within the one-year study period.
3.1. Statistical Analysis
For data analysis, the normality of continuous variables (e.g., maternal age, Apgar scores) was assessed using the one-sample Kolmogorov-Smirnov (K-S) test. If normality was confirmed, the independent samples t-test was applied for comparisons between groups. For categorical variables (e.g., NICU admission, LBW, congenital anomalies), the chi-square test was used. If expected frequencies in any cell were < 5, Fisher’s exact test was performed instead. All tests were conducted using IBM SPSS Statistics (version 26) software, with a significance level set at less than 0.05.
4. Results
In this study, 180 pregnant women were divided into case (n = 90) and control (n = 90) groups, respectively infected and not infected with COVID-19, to determine the frequency of complications in neonates born to mothers infected with COVID-19.
4.1. Maternal Demographics
According to Table 1, the demographic characteristics of the mothers in both the COVID-19 group and the control group were largely similar, with no significant differences in age or gravida. The mean ± standard deviation (SD) age of the pregnant women with COVID-19 was 31.46 ± 5.56 years, and that of the controls was 30.76 ± 5.84 years (P = 0.309).
| Variables | Covid-19 Mothers (N = 90) | Control Group (N = 90) | P-Value |
|---|---|---|---|
| Age | 31.46 ± 5.56 | 30.76 ± 5.84 | 0.309 |
| Neonatal sex | 0.289 | ||
| Male | 41 (45.56) | 33 (36.67) | |
| Female | 49 (54.44) | 57 (63.33) | |
| Mode of delivery | 0.221 | ||
| Cesarean section | 51 (56.67) | 59 (65.56) | |
| Normal vaginal delivery | 39 (43.33) | 31 (34.44) | |
| Gravida | 2.29 ± 2.00 | 2.67 ± 1.97 | 0.198 |
| Trimester of pregnancy at infection | - | ||
| First trimester | 22 (24.44) | - | |
| Second trimester | 26 (28.89) | - | |
| Third trimester | 42 (46.67) | - |
a Values are expressed as No. (%) or mean ± SD.
4.2. Neonatal Outcomes
As shown in Table 2, neonatal outcomes were markedly worse in the COVID-19 group compared to the control group. The NICU admission was significantly more common among infants born to COVID-19-positive mothers (25.56% vs. 0%, P < 0.001). The incidence of LBW was also significantly higher in the COVID-19 group (28.89% vs. 6.67%, P < 0.001). Neonatal respiratory symptoms were more frequent in the COVID-19 group (69.23% vs. 30.77%, P = 0.007), and there was a higher rate of congenital anomalies, although this was not statistically significant (7.78% vs. 3.33%, P = 0.193). Additionally, there were notable differences in gestational age at delivery. The COVID-19 group had a higher rate of preterm births (< 37 weeks) at 32%, compared to 20% in the control group (P = 0.048). Apgar scores were significantly lower in the COVID-19 group, both at 1 minute (7.57 ± 2.53 vs. 8.87 ± 0.34; P < 0.001) and at 5 minutes (8.84 ± 2.13 vs. 9.87 ± 0.34; P < 0.001). Although mortality rates were higher in the COVID-19 group, this difference was not statistically significant (P = 0.051). Mortality rates in each group were calculated by dividing the number of neonatal deaths within 30 days of birth by the total number of neonates in the respective group (n = 90). For the case group, 8 neonates died, resulting in a mortality rate of 8.89%, and for the control group, 3 neonates died, resulting in a mortality rate of 3.33%.
| Clinical Characteristics | Infected Mothers | Healthy Mothers | P-Value b |
|---|---|---|---|
| NICU admission | < 0.001 | ||
| Yes | 23 (25.56) | 0 (0) | |
| No | 67 (74.44) | 90 (100) | |
| LBW | < 0.001 | ||
| Yes | 26 (28.89) | 6 (6.67) | |
| No | 64 (71.11) | 84 (93.33) | |
| Congenital anomaly | 0.193 | ||
| Yes | 7 (7.78) | 83 (92.22) | |
| No | 3 (3.33) | 87 (96.67) | |
| Neonatal respiratory symptoms | 0.007 | ||
| Yes | 27 (69.23) | 12 (30.77) | |
| No | 63 (44.68) | 78 (55.32) | |
| Gestational age (wk) | 0.048 | ||
| < 37 (term) | 58 (64.44) | 70 (77.78) | |
| > 37 (preterm) | 32 (35.56) | 20 (22.22) | |
| Apgar score | |||
| 1st min of life | 7.57 ± 2.53 | 8.87 ± 0.34 | < 0.001 |
| 5th min of life | 8.84 ± 2.13 | 9.87 ± 0.34 | < 0.001 |
Abbreviations: NICU, neonatal intensive care unit; LBW, low birth weight.
a Values are expressed as No (%) or mean ± SD.
b P-value ≤ 0.05 is considered statistically significant.
4.3. Impact of COVID-19 Infection Timing
Our results showed that the timing of COVID-19 infection during pregnancy appeared to influence neonatal outcomes. As presented in Table 3, infant mortality was highest among mothers infected during the third trimester (14.28%), although this finding was not statistically significant (P = 0.157). Congenital anomalies were most common when infection occurred in the second trimester (85.71%, P = 0.002). Neonatal respiratory symptoms were more prevalent when mothers were infected in the third trimester, though the differences were not statistically significant (P = 0.654).
| Trimester of Maternal COVID-19 Infection | Yes | No | P-Value b |
|---|---|---|---|
| Infant mortality | 0.157 | ||
| 1st | 0 (0) | 22 (100) | |
| 2nd | 2 (7.69) | 24 (92.31) | |
| 3rd | 6 (14.28) | 36 (85.72) | |
| Total | 8 (8.89) | 82 (91.11) | |
| Congenital anomaly | 0.002 | ||
| 1st | 0 (0) | 22 (26.50) | |
| 2nd | 6 (85.71) | 20 (24.09) | |
| 3rd | 1 (14.29) | 41 (49.39) | |
| Total | 7 (100) | 83 (100) | |
| Neonatal respiratory symptoms | 0.654 | ||
| 1st | 7 (31.81) | 15 (68.18) | |
| 2nd | 6 (23.07) | 20 (76.92) | |
| 3rd | 14 (43.75) | 28 (66.67) | |
| Total | 27 (30) | 63 (70) |
a Values are expressed as No (%) or mean ± SD.
b P-value ≤ 0.05 is considered statistically significant.
4.4. Congenital Malformations
An analysis of congenital malformations revealed a significant association with maternal age and neonatal sex (Table 4). Infants born to mothers aged 31 - 40 years had a higher incidence of congenital anomalies (70%) compared to other age groups (P = 0.54). Female infants were more likely to have congenital anomalies (90%) compared to males (10%), a difference that was statistically significant (P = 0.04). Moreover, no significant relationship was observed between maternal pregnancy diseases and congenital anomalies (P = 0.425).
| Variables | Congenital Anomaly | P-Value b | |
|---|---|---|---|
| Yes | No | ||
| Maternal age | 0.54 | ||
| < 20 | 0 (0) | 2 (1.17) | |
| 21 - 30 | 3 (30) | 74 (43.52) | |
| 31 - 40 | 7 (70) | 80 (47.05) | |
| 41 - 50 | 0 (0) | 11 (6.47) | |
| Neonatal sex | 0.04 | ||
| Boy | 1 (10) | 73 (42.94) | |
| Girl | 9 (90) | 97 (57.05) | |
| Maternal pregnancy diseases | 0.425 | ||
| Present | 4 (7.69) | 48 (92.31) | |
| Absent | 6 (4.68) | 122 (95.32) | |
a Values are expressed as No (%) or mean ± SD.
b P-value ≤ 0.05 is considered statistically significant.
5. Discussion
Considerable attention has been directed toward the impact of the COVID-19 pandemic on pregnant women, who face high risks due to their increased susceptibility to respiratory infections and severe pneumonia. The interaction of COVID-19 with pregnancy-specific immune and anatomical changes may elevate the risk of infection and subsequently influence both immune responses and maternal-neonatal health outcomes. Previous studies on other CoVs, such as SARS, have established their association with severe perinatal complications, including miscarriage, IUGR, and congenital disorders (13).
Despite the global focus on COVID-19, there remains a significant gap in understanding its specific effects during pregnancy, particularly in underrepresented populations. This study aimed to address this gap, providing crucial insights into the complications associated with SARS-CoV-2 infection during pregnancy in Sistan and Baluchestan province, Iran. The findings of this study showed the effect of COVID-19 on maternal and neonatal outcomes, emphasizing challenges faced by pregnant women during the pandemic. The significant differences observed in gestational age at delivery, NICU admissions, and the prevalence of LBW among infants born to COVID-19-positive mothers reveal the extent to which the virus can complicate pregnancy.
Our findings revealed that COVID-19 during pregnancy significantly increased the risk of preterm birth, NICU admission, LBW, and lower Apgar scores, aligning with similar studies. We also observed more frequent neonatal respiratory symptoms in the COVID-19 group. Our study identified the timing of infection as a critical factor, particularly with congenital anomalies associated with second-trimester infections. These results not only emphasize the vulnerability of this population but also raise critical questions about the long-term implications for both mothers and their newborns. Understanding these outcomes is essential for informing clinical practices and public health strategies to protect pregnant women in the face of ongoing and future pandemics.
Some studies have demonstrated that patients in the acute phase of COVID-19 may face cytokine release syndrome (CRS). Viral infections can cause dysfunction or failure of multiple organs. Pulmonary involvement with higher pro-inflammatory interleukins (ILs) and tumor necrosis factor alpha (TNF-α) are associated with mortality. On the other hand, pregnancy has been shown to aid the body’s immune system in reaching modulation. Gonadotropin (GNT) and progesterone (PGT) inhibit the T1 pro-inflammatory pathway through TNF-α reduction, and it has been reasoned that the modulated immune system may protect pregnant women against CRS and its associated mortality. Therefore, pregnant women may be at fewer risks, although neonatal complications can be seriously detrimental (14).
Based on the study findings, the 1- and 5-minute Apgar scores of the neonates born to COVID-19-positive mothers were significantly lower in the case group than in the controls. Li et al. found in their case-control study on maternal-neonatal outcomes in pregnant women with COVID-19 that the 1-minute Apgar score was significantly lower in the case group than in the control group (15). In a prospective cohort study on congenital COVID-19 infection, Antoun et al. reported that 29% of the cases contracted the disease and established that the 1-, 3-, and 5-minute Apgar scores were significantly lower than those in the control group, consistent with the present study (16).
The frequency of gestational age below 37 weeks (preterm), NICU admission, LBW, and respiratory symptoms in pregnant women with COVID-19 were higher than those in the controls. As a result, the mortality rate in neonates born to COVID-19-positive mothers was higher than in the control group, indicating a borderline significant difference. Vilar et al. investigated maternal-neonatal complications and mortality rates among pregnant women and a control group in a multinational cohort study. Their findings showed that COVID-19 increased the probability of preterm birth (59%), fetal distress (70%), NICU admissions (97%), and birth weight below 2500 g (58%) but decreased gestational age (39%) (17). Additionally, Li et al. verified that the frequency of LBW and preterm birth in neonates born to mothers infected with COVID-19 was significantly higher than in the control group (15).
Mullins et al. reported that the most common maternal outcomes associated with COVID-19 infection were fetal distress, premature rupture of membranes (PROM), and preterm delivery. Notably, 42% of mothers infected with the virus gave birth prematurely (18). Similarly, Gupta et al. found an association between COVID-19 infection and an increase in preterm births, suggesting that the use of antiviral drugs, which can stimulate labor, may contribute to this outcome (19). Additionally, Azh et al. reported that higher rates of preterm delivery are associated with increased rates of infant mortality (20). They found that infants born to mothers infected with COVID-19 had a significant incidence of poor neonatal outcomes, including respiratory distress, fever, tachycardia, gastrointestinal bleeding, nutritional intolerance, and milk regurgitation.
Alserehi et al. demonstrated that the most common neonatal complications were prematurity, low gestational age, fetal distress, LBW, and bacterial pneumonia. Their study also revealed that 50% of these infants required NICU hospitalization, 23% needed mechanical ventilation, and 57% experienced spontaneous abortions. Most of the mothers in this study were infected during the first trimester, and 80% of the infants were born prematurely. These findings suggest that infants with underlying conditions and those born before 37 weeks are at higher risk of severe disease caused by COVID-19 (21).
The study findings did not reveal a significant difference in congenital disorders with respect to COVID-19 infection and non-infection. Nevertheless, these abnormalities were more common in the second trimester. This discrepancy highlights the importance of increased care during different time periods to avoid contracting COVID-19. During this period, the rapid development of fetal organs could trigger problems; therefore, it is crucial to prevent and manage COVID-19 infection to minimize complications.
In our previous study, we reported a 37% prevalence of preterm birth and a significant incidence of complications such as acute respiratory distress syndrome (21%) and stillbirth (10%) among pregnant women with COVID-19. The present study found a slightly lower preterm birth rate of 32%, though neonatal outcomes such as NICU admissions (25.56%), LBW (28.89%), and respiratory symptoms (69.23%) were notably higher. Furthermore, our earlier research documented a cesarean section rate of 30% among COVID-19-positive pregnancies, whereas the current study shows an increased rate of 56.67%, potentially reflecting changes in clinical management or differing population characteristics (12).
Both studies illustrate the considerable risks posed by COVID-19 during pregnancy, but the current study provides additional insights into the timing of infection, with second-trimester infections showing a significant association with congenital anomalies (P = 0.002), a factor not evaluated in the earlier work. Together, these findings confirm the expanding understanding of maternal and neonatal risks correlated with COVID-19.
Similarly, a case-control study in southern Iran reported higher rates of vaginal bleeding, fetal distress, preterm birth, intrauterine death, ICU admission, LBW, and NICU admission among COVID-19-infected pregnant women. While neonatal transient tachypnea, pneumonia, and abnormal lung X-rays were more common, these differences were not statistically significant (22). These findings align with our results, highlighting the need for enhanced prenatal monitoring and pregnancy-specific interventions to improve maternal and neonatal outcomes.
5.1. Conclusions
The study demonstrates that COVID-19 during pregnancy is associated with increased risks of preterm birth, lower Apgar scores, NICU admissions, LBW, and neonatal respiratory symptoms. The timing of infection during pregnancy appears to play a crucial role in determining neonatal outcomes, particularly regarding congenital anomalies and infant mortality. Further research is needed to explore the mechanisms behind these associations and to develop strategies for mitigating these risks in pregnant women affected by COVID-19.
5.2. Limitations
There were some limitations in this study. First, there was no access to all medical records, which was addressed by obtaining permission from the Ethics Committee and providing it to the relevant officials. Second, inaccuracies in the review of medical records could affect the study results. To address this, a medical student informed of the conditions and knowledgeable about the study objectives supervised data collection alongside the researcher. Third, the incompleteness of medical records in terms of the information needed for the present study was also a limitation, so such cases were excluded.