1. Background
Hospital-acquired infections (HAIs) manifest during receiving medical care, typically developing in a hospital or other healthcare setting, and are defined as those that emerge 48 hours or more after admission or within 30 days following healthcare interventions (1). They are associated with high rates of morbidity and mortality in hospitals globally. These infections also contribute to higher hospital costs due to prolonged hospital stays and escalate antimicrobial resistance rates (2, 3). Hospital-acquired infections are mainly associated with urinary tract infections (UTIs), respiratory tract infections (RTIs), infections of the circulatory system, and surgical site infections (4). According to World Health Organization (WHO) data, nosocomial infections occur in 15% of patients in developing countries and 7% in developed countries (5). Bacteria are responsible for most nosocomial infections; however, other microbes, such as protozoans, fungi, and viruses, are less common (6). These infections are controlled by antibiotic treatment. However, injudicious antibiotic use has led to an increase in drug-resistant bacteria, resulting in the emergence of significant threats to patients' health (7). The WHO has released a list of antibiotic-resistant "priority pathogens" categorized into critical, high, and medium priority groups. The critical group comprises gram-negative multidrug-resistant (MDR) bacteria, such as Enterobacterales (Klebsiella pneumoniae, Escherichia coli), Acinetobacter baumannii, and Pseudomonas aeruginosa, which have resisted commonly used antibiotics (8). The studies conducted in Iran show that the prevalence and antibiotic resistance pattern of nosocomial infections varies in Iranian hospitals (9-12). Therefore, monitoring drug resistance patterns of nosocomial infections in hospitals is crucial in healthcare management (13). The primary goal of the infection prevention and control program is to eliminate nosocomial infections. However, it is still necessary to conduct epidemiological surveillance to demonstrate performance improvements and achieve this goal (14).
2. Objectives
This study aims to summarize the surveillance results of prevalence and antibiotic susceptibility of gram-negative HAIs isolated from patients referred to a tertiary hospital for 5 months.
3. Methods
This study was approved by the local Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1402.376). Between October 1, 2022, and February 28, 2023, the study was conducted at a tertiary hospital affiliated with Tehran University of Medical Sciences (Yas Hospital). The study included all confirmed patients with nosocomial infections, such as UTIs, central line-associated bloodstream infections, surgical site infections, ventilator-associated pneumonia, and hospital-acquired pneumonia. Specimens from outpatients and the emergency ward were excluded from the study. All specimens, including midstream urine, blood, respiratory specimens (bronchial lavage fluid and tracheal secretions), surgical wound samples, and other specimens from patients admitted to the coronary care unit (CCU), intensive care unit (ICU), neonatal intensive care unit (NICU), as well as general and surgical wards, were collected according to standard guidelines and transported to the hospital laboratory (15). Afterward, bacterial identification was conducted through colony characteristics, gram staining, and various biochemical tests following established protocols (16). The antibiotic susceptibility test was measured by the Kirby-Bauer disk diffusion method according to the clinical and laboratory standards institute (CLSI) guidelines, and the resistance rate was reported according to the guideline criteria (17). The antimicrobial agents (Padtanteb, Tehran, Iran) included in this study were gentamicin (GM; 10 μg), ciprofloxacin (CIP; 5 μg), cefepime (FEP; 30 μg), ceftazidime (CAZ; 30 μg), cefotaxime (CTX; 30 μg), imipenem (IPM; 10 μg), meropenem (MR; 10 μg) and piperacillin/tazobactam (TZP; 100/10 μg). The E. coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality controls. The isolates that were resistant to at least one potentially effective antimicrobial drug in three or more classes (such as penicillin/cephalosporins, fluoroquinolones, carbapenems, and aminoglycosides) were considered MDR strains (18, 19).
3.1. Statistical Analysis
Data were analyzed using SPSS software version 22. Nominal variables were displayed as frequencies and percentages. Chi-square and Fisher's exact test assessed the significant difference between antibiotic resistance, wards, clinical samples, and gram-negative bacteria. P-values < 0.05 were considered statistically significant.
4. Results
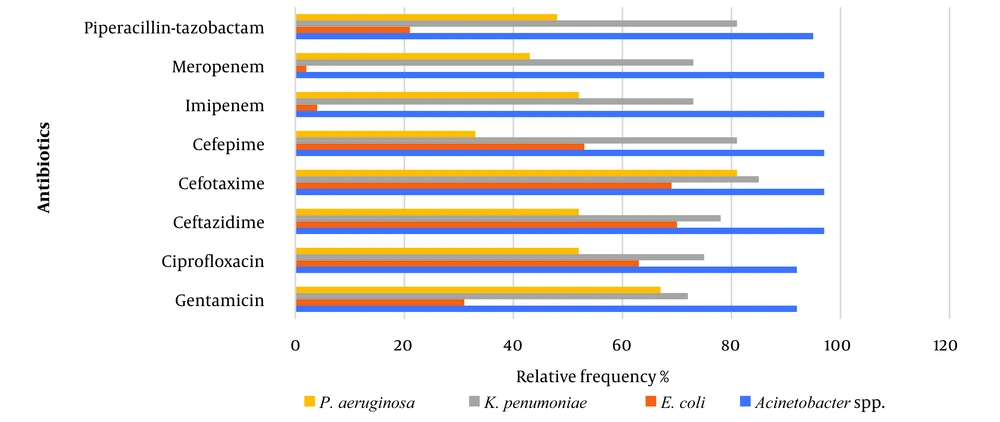
A total of 260 gram-negative nosocomial isolates were collected over five months. Among these, 140 isolates (53.8%) were obtained from the ICU, followed by 45 isolates (17.3%) from the surgery ward, 35 isolates (13.5%) from the general ward, 26 isolates (10%) from the CCU, and 14 isolates (5.4%) from the NICU. Table 1 presents the distribution of gram-negative bacteria isolated from various samples. Most isolates, 98 (37.7%), were obtained from urine samples. Following this, 92 (35.4%) were isolated from respiratory infections, 47 (17.3%) from blood samples, and 17 (6.5%) from surgical wounds. The lowest number of isolates, 8 (3.1%), was found in other specimens. Table 1 indicates that this study’s most commonly identified pathogens were K. pneumoniae (N = 132, 50.8%), followed by E. coli (N = 71, 27.3%) and Acinetobacter spp. (N = 36, 13.8%), and P. aeruginosa (N = 21, 8.1%). Most bacteria isolated from blood, respiratory, surgical, wound, and other specimens were identified as K. pneumonia. However, E. coli was the most frequently isolated in UTIs (P-value = 0.005). Figure 1 illustrates the antimicrobial susceptibility patterns of the isolated bacterial pathogens. Among gram-negative bacteria, Acinetobacter spp. exhibited the highest resistance rates to all antibiotics tested (P-value = 0.001). Specifically, it demonstrated a prevalence of 97% resistance to carbapenems and cephalosporins, 95% to TZP, and 92% to both CIP and GM. Subsequently, K. pneumoniae exhibited resistance between 70% and 85% to antibiotics. In contrast, 96% and 98% of E. coli isolates displayed sensitivity to IPM and MR, respectively. Notably, Figure 1 shows that E. coli isolates had the lowest resistance rates to TZP and GM (21% - 31%) (P-value = 0.006). Depending on the tested antimicrobial, the resistance rates for P. aeruginosa isolates ranged from 33% to 81%. Moreover, the most frequent MDR strain was Acinetobacter spp. (35/36, 97%), followed by K. pneumoniae (98/132, 74%), P. aeruginosa (9/21, 43%) and E. coli (24/71, 34%) (P-value = 0.001). As shown in Table 2, the findings further revealed that strains isolated from the ICU exhibited the highest resistance to antibiotics (P-value = 0.0045). Notably, isolates from the surgery ward had the lowest resistance rates to carbapenems and TZP (8.9% - 17.8%). Moreover, all isolates from the NICU were sensitive to CIP. Multidrug-resistant strains were also most prevalent in the ICU, followed by those from the general ward and CCU (P-value = 0.003).
| Specimens | Number of Isolates (%) | Total (%) | |||
|---|---|---|---|---|---|
| Acinetobacter baumannii Spp. | Escherichia coli | Klebsiella pneumoniae | Pseudomonas aeruginosa | ||
| Blood | 9 (25) | 6 (8.5) | 27 (20) | 3 (14) | 45 (17.3) |
| Respiratory a | 20 (55) | 3 (4) | 63 (48) | 6 (28) | 92 (35.4) |
| Urine | 6 (17) | 54 (76) | 28 (21) | 10 (48) | 98 (37.7) |
| Surgical wound | 1 (3) | 6 (8.5) | 9 (7) | 1 (5) | 17 (6.5) |
| Other | 0 (0) | 2 (3) | 5 (4) | 1 (5) | 8 (3.1) |
| In total | 36 (100) | 71 (100) | 132 (100) | 21 (100) | 260 (100) |
a Respiratory = bronchial lavage fluid and tracheal secretion.
| Antibiotics | Number of Isolates (%) | Total (%) | ||||
|---|---|---|---|---|---|---|
| CCU | ICU | General | NICU | Surgery | ||
| GM | 13 (50) | 111 (79) | 18 (51.4) | 11 (78) | 11 (24) | 164 (63) |
| CIP | 16 (61) | 125 (89) | 23 (66) | 0 (0) | 24 (53) | 188 (72) |
| FEP | 17 (65) | 121 (86) | 22 (63) | 8 (57) | 19 (42) | 187 (72) |
| CAZ | 19 (73) | 130 (93) | 28 (80) | 9 (64) | 25 (55) | 211 (81) |
| CTX | 19 (73) | 129 (92) | 31 (88) | 11 (79) | 24 (53) | 214 (82) |
| IPM | 6 (23) | 117 (83) | 14 (40) | 4 (28) | 5 (11) | 146 (56) |
| MR | 6 (23) | 116 (83) | 12 (34) | 4 (28) | 4 (9) | 142 (54) |
| TZP | 11 (42) | 118 (84) | 20 (57) | 9 (64) | 8 (18) | 166 (64) |
| MDR | 13 (50) | 120 (86) | 18 (51) | 4 (28) | 11 (24) | 166 (64) |
Abbreviation: MDR, multidrug-resistant; CCU, coronary care unit ; ICU, intensive care unit; NICU, neonatal intensive care unit; GM, gentamicin; CIP, ciprofloxacin; FEP, cefepime; CAZ, ceftazidime; CTX, cefotaxime; IPM, imipenem; MR, meropenem; TZP, piperacillin-tazobactam.
5. Discussion
Hospital-acquired infections are a significant healthcare issue that causes economic losses and impacts productivity. They result in prolonged hospital stays, long-term disabilities, financial burdens on healthcare systems, antimicrobial resistance, increased costs for patients and families, and higher patient mortality rates (20). According to the present study, the overall rate and prevalence of antibiotic resistance and MDR isolates in HAIs were more prevalent in the ICU than in other wards, which aligns with previous findings (12, 21-24). This can be attributed to the increased susceptibility of patients admitted to ICU to nosocomial infections compared to individuals in general wards. This increased susceptibility may be due to the frequent utilization of invasive medical devices among ICU patients and their heightened exposure to a greater diversity of antibiotic-resistant pathogens (25). Conversely, the lowest prevalence of nosocomial infections, accounting for 5.4% of total infections, was observed in the NICU, where no resistance to CIP was noted. This finding may be related to the off-label use of CIP in this population, partly due to the limited pharmacokinetic studies available for neonates (26). Regarding HAIs in terms of the type of infection, UTI was the most common type, accounting for 37.7% of all healthcare-associated infections. This confirms reports by other authors that have mentioned UTI consistently emerging as the leading cause of nosocomial infection (11, 27-29). The high prevalence of this infection can be associated with the use of medical instruments such as nephrostomy tubes, ureteric stents, suprapubic tubes, or Foley catheters (30). In contrast, the prevalence of surgical wound infections was among the lowest observed in the study, and these infections also exhibited the lowest rate of carbapenem resistance. This finding suggests that patients likely did not acquire these infections within the hospital environment. Instead, it may indicate that the surgical procedures were effectively managed with appropriate infection control measures. Additionally, the lower incidence of resistance in these infections may reflect effective prophylactic antibiotics and adherence to best practices in surgical care, further supporting the notion that these infections were acquired outside the hospital setting (31). The analysis based on the type of microorganism indicated that K. pneumoniae was identified as the predominant bacterium overall, while E. coli emerged as the primary microorganism among patients with UTIs. These results are comparable with those of other studies (32, 33). A similar study in Iran indicated that E. coli and K. pneumoniae were the most common infectious agents in UTI and respiratory samples, respectively (29). Another study by Tolera et al. elucidated that E. coli exhibited the highest prevalence among isolated gram-negative bacteria. Surprisingly, K. pneumoniae was primarily identified in cases of RTIs, whereas E. coli prevailed as the most common microorganism in UTIs (34). The high propensity of E. coli to trigger UTIs can be attributed to its virulence factors. This microorganism produces numerous adhesins that facilitate bacterial adherence to host cells. Moreover, the production of various toxins by E. coli influences the immune response, and the presence of siderophores aids in iron uptake (35, 36). Although K. pneumoniae was the most prevalent bacteria, accounting for 50.8% of total isolated strains, A. baumannii showed a significantly higher level of antibiotic resistance, ranging from 92% to 97%. These findings are consistent with the results of a study by Zhang et al. in 2011, which indicated that although K. pneumoniae was more common, A. baumannii showed more excellent resistance to several antibiotics, including TZP, IPM, MR, CAZ, CTX, and FEP (37). The prevalent multidrug resistance pattern observed in A. baumannii clinical isolates has established carbapenems as frequently recommended treatment choices for Acinetobacter infections in healthcare settings (38). However, this study revealed a high prevalence of carbapenem resistance among A. baumannii isolates, with rates just below 90% for IPM and MR, corroborating the findings of other studies (38-41). The rise in carbapenem utilization has led to the widespread emergence of carbapenem-resistant A. baumannii strains, presenting a substantial risk to global health and patient safety due to the ongoing reduction in available treatment modalities (38, 42). Given the alarming prevalence of antibiotic resistance within the healthcare sector, the implementation of stringent disinfection protocols for patient environments, meticulous screening of high-risk individuals, effective management of infection reservoirs, enhancement of routine hygiene practices, and the judicious prescription of narrow-spectrum antibiotics stand as imperative strategies to combat the dissemination of MDR strains and proactively avert potential outbreaks (43, 44). In this study, E. coli was identified as the second most prevalent strain, mostly isolated from UTI patients. However, the incidence of carbapenem resistance was not as high as that of A. baumannii and K. pneumoniae isolates. According to the results, supported by Chen et al.'s findings, E. coli isolates exhibited the most resistance to CIP and third-generation cephalosporins (45). The resistance observed can be attributed to the frequent use of fluoroquinolones or third-generation cephalosporins in hospital settings to provide empiric coverage for this pathogen in UTI patients. Consequently, the high prevalence of antibiotic resistance can compromise treatment efficiency by limiting available treatment options (46-48). As part of the study, P. aeruginosa was identified as the least prevalent bacterium isolated from patients. However, the fact that over 40% of these isolates exhibited carbapenem resistance raises significant concerns within the healthcare sector. Notably, the rate of carbapenem resistance in P. aeruginosa varies by geographical region, ranging from 10% to 50%. This variability is particularly alarming, as carbapenems are among the most effective antimicrobial agents for treating severe infections. Their role as one of the last-resort treatment options for MDR bacterial infections further underscores the urgency of addressing this growing resistance issue (49, 50). The study had several limitations as it was limited to a single territory hospital within the province, potentially impacting the generalizability of the results. Therefore, it is advisable to replicate the study in other cities or hospitals within the province for a more comprehensive understanding. Additionally, the study focused solely on four types of nosocomial infections. Therefore, it is suggested that future studies explore additional types of nosocomial infections to provide a more comprehensive analysis. Another limitation of the study is the lack of patient follow-up post-discharge. Hospitals need to implement structured and systematic monitoring of nosocomial infections, particularly in high-risk wards, focusing on older patients and those with multiple risk factors for nosocomial infections.
5.1. Conclusions
This study found a high prevalence of nosocomial infections among patients admitted to hospital wards, particularly in ICU settings. There was a significant degree of resistance to the treating antibiotics in the bacteria that caused the infections in the ICU. Urinary tract infection was the most common hospital infection in the study. Additionally, it is noteworthy that A. baumannii displayed a higher level of phenotypic resistance than other bacteria. It suggests that to address HAIs and antibiotic resistance effectively; hospitals should continuously monitor MDR isolates, especially within the ICU, so that the infection does not spread to other wards of the hospitals, particularly those that are antibiotic-resistant, enforce strict infection control measures, manage resistant pathogens like A. baumannii, and promote responsible antibiotic use through education and stewardship.