1. Background
Meningoencephalitis is a life-threatening clinical condition, particularly associated with significant mortality and morbidity in childhood (1). Viruses can infect the skin, respiratory tract, gastrointestinal tract, or urinary tract, typically spreading to the central nervous system (CNS) through the blood or nerves. The blood route is the more common pathway for transmission. Infections of the CNS can manifest as meningitis, encephalitis, or, occasionally, a combination of both, known as meningoencephalitis, frequently observed in children (2). Viral meningitis is detected by the absence of bacterial growth in cultures and an increased white blood cell count in the cerebrospinal fluid (CSF), where lymphocytes predominate. Annually, 7.6 per 100,000 adults are affected by meningitis, with viral pathogens being the most common cause (1). The global prevalence of bacterial meningitis has decreased due to vaccination; however, viral meningitis remains common, posing a major public health concern (3). Generally, meningitis is clinically characterized by fever, headache, photophobia, and neck stiffness, often accompanied by nausea and vomiting (4). Encephalitis, which is the inflammation of the brain parenchyma, is also typically caused by viral pathogens. Viral encephalitis is an acute clinical condition characterized by changes in mental status, such as lethargy, headache, decreased consciousness, convulsions, and fever over 38°C (5). The yearly incidence of encephalitis in children is approximately 16 cases per 100 children in their second year of life. This rate remains high until age 10, then decreases to around 1 case per 100,000 children annually by age 15 (6).
Infection with a virus in the CNS can lead to severe inflammation in distinct body areas, including the meninges, brain tissue, and cranial nerves, or in multiple regions simultaneously (7-9). Meningitis occurs when the inflammation is confined to the meninges, while encephalitis results from brain tissue involvement. Pathologically, a continuous and potentially life-threatening inflammatory process can occur between these adjacent anatomical regions, known as meningoencephalitis (10, 11). Enteroviruses, the mumps virus, and herpes simplex virus (HSV) are the primary culprits behind viral meningoencephalitis, contributing significantly to the global burden of this condition (12-14). These viruses account for 23 - 61%, 7.5 - 15.8%, and 0.5 - 18% of viral meningitis cases annually (14, 15). Meanwhile, other viruses like varicella-zoster virus (VZV), West Nile virus (WNV), and cytomegalovirus (CMV) are responsible for sporadic encephalitis cases worldwide (16, 17).
2. Objectives
This study was conducted in the northeastern region of Iran, where researchers collected CSF samples from three tertiary university hospitals. The samples were obtained from children under 14 who exhibited symptoms related to meningitis and encephalitis. The specific hospitals involved in the sample collection were Imam Reza Bojnourd and Sheikh Mashhad hospitals. The primary aim of the study was to assess the presence of various viral pathogens in the CSF of these pediatric patients, including enteroviruses, VZV, mumps virus, WNV, HSV, adenovirus, CMV, and poliovirus.
3. Methods
3.1. Study Design
This cross-sectional study collected 120 samples from children under 14 at Imam Rezai Bojnourd and Sheikh Mashhad hospitals. The inclusion criteria for this study included children who exhibited one or more symptoms associated with meningitis and encephalitis, such as headache, fever, seizures, nausea, vomiting, or neck stiffness. Exclusion criteria of our study include issues such as lysis of the CSF sample and lack of suitable laboratory conditions for performing laboratory tests. This study included children hospitalized with the aforementioned symptoms from November 2021 to July 2022. Relevant data were extracted from patients' files. Initial routine bacterial culture tests were conducted following the Clinical and Laboratory Standards Institute guidelines to identify bacterial pathogens. Samples that tested negative for bacterial presence were preserved at -70°C for potential future investigations.
3.2. Nucleic Acid Extraction
According to the manufacturer's instructions, genomic DNA and RNA were isolated from CSF samples using the QIAGEN viral DNA and RNA extraction kit. The quality of the extracted nucleic acids was assessed using a spectrophotometer. The extracted RNA was utilized for cDNA synthesis, while the nucleic acids were stored frozen for PCR assay. The extracted DNA should be stored in a refrigerator at 4°C. Viral DNA can be stored in a freezer at -20°C, but freezing may cause DNA breakage. The extracted RNA is best stored in a freezer at -20°C. The selection of the B-globin gene as the internal control was a crucial step in the process.
3.3. Primer Design
The primer design involved using software to target conserved regions within the viral genomes. The optimal thermal program was established through gradient PCR assays. Table 1 lists the specific primers utilized in each panel. For both panels, the thermal cycles included a denaturation step, followed by 30 cycles of denaturation at 94°C for 30 seconds, annealing at 57°C for 45 seconds, and extension at 72°C for 30 seconds.
| Variables | Sequence | Product Size (bp) |
|---|---|---|
| VZV | 5- GTGCTGGGAGGAATTGTTACAG-3 | 186 |
| 5- TCGTCGCTATCGTCTTCACCAC-3 | ||
| Mump | 5- TCTCACCCATAGCAGGGAGTTATAT -3 | 79 |
| 5- GTTAGACTTCGACAGTTTGCAACAA -3 | ||
| Pol 1, 2 | 5- CAGTTCAAGAGCAARCACC -3 | 194 |
| 5- TCRTCCATRATMACYACWCC -3 | ||
| CMV | 5- TGAACATCCCCAGCATCAACG -3 | 137 |
| 5- CAGTCCCGAGACCGTGAGAC -3 | ||
| Adeno | 5- GCCGCGGATGTCAAAGT -3 | 291 |
| 5- CAGTGGTCTTACATGCACATC -3 | ||
| HSV | 5- CGCTGGTCATTACCACC -3 | 125 |
| 5- CGAGCCGATGACTTACT -3 |
Primers Used in This Study
3.4. Polymerase Chain Reaction
The PCR amplification was carried out using a TAKARA Gradient PCR thermal cycler with a 50 µl volume. We utilized Emerald Amp® MAX PCR Master Mix (Takara, Japan) for all PCR reactions. Gene specificity was confirmed using NCBI BLAST, and the primers were designed using Mega 4, Allele ID6 software, and Oligo 6. PCR and RT-PCR were conducted for DNA and RNA viruses, respectively. Reactions were performed in a 20 µl volume following this program: Initial enzyme activation at 94°C for 5 minutes, followed by 40 cycles of amplification (30-second denaturation at 94°C, 45-second annealing at 57°C, and 30-second extension at 72°C), and a final extension at 72°C for 5 minutes. Subsequently, electrophoresis was performed on a 1.5% agarose gel to visualize the obtained amplicons, which were stained with ethidium bromide (0.5 μg/ml) and visualized under UV light.
3.5. Multiplex PCR
Two separate multiplex PCR assays were developed to detect the prevalent viral pathogens responsible for meningoencephalitis. Panel 1 targeted enterovirus, HSV, poliovirus types 1 and 2, and poliovirus types 3 and 4, while panel 2 focused on CMV, VZV, adenovirus, and WNV. Subsequently, the PCR products underwent gel electrophoresis for analysis. Positive controls obtained from the Lab of Day Hospital in Tehran, Iran, were used to ensure quality control of the assay.
3.6. Multiplex PCR
Two separate multiplex PCR assays were developed to detect the prevalent viral pathogens responsible for meningoencephalitis. Panel 1 targeted enterovirus, HSV, poliovirus types 1 and 2, and poliovirus types 3 and 4, while panel 2 focused on CMV, VZV, adenovirus, and WNV. Subsequently, the PCR products underwent gel electrophoresis. The assay's positive controls from the Lab of Day Hospital in Tehran, Iran, ensured quality control.
4. Results
In the study period, 120 CSF samples with negative bacterial culture results were tested for possible viral viruses causing meningoencephalitis. The initial diagnosis of these conditions relied on clinical assessments, followed by laboratory evaluations of routine CSF culture and biochemistry. The samples were obtained from Mashhad and Bojnourd's main children's university hospitals. Routine CSF culture showed that 8 out of the 128 samples (6.25%) referred to the microbiology lab were positive for bacterial pathogens. The 8 bacterial CSF infections consisted of examinations conducted on the samples revealed that there were 2 isolates of Escherichia coli, 3 isolates of pneumococcus, and one isolate each of Neisseria, Enterococcus, and Staphylococcus aureus bacteria identified in the patient's CSF.
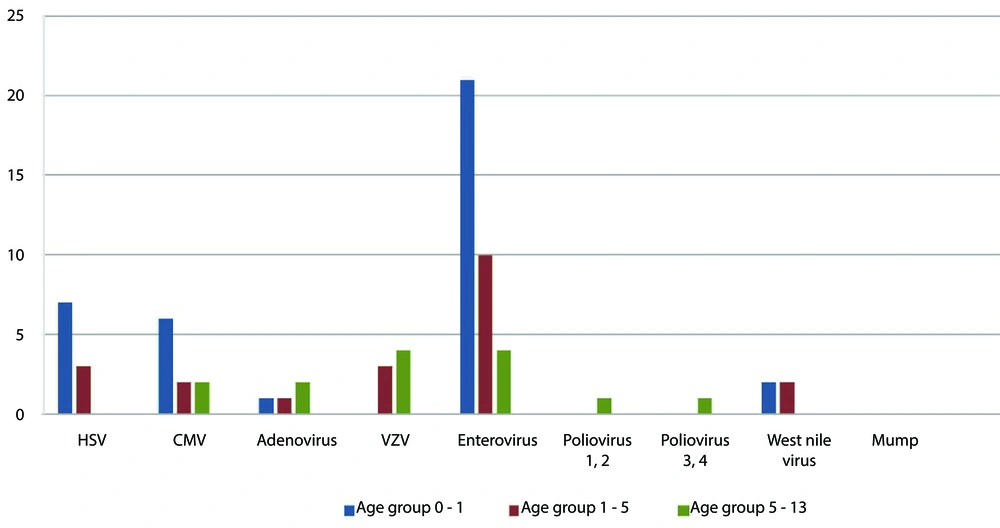
As shown in Table 2, out of the 120 samples negative for bacterial culture, 72 (60%) cases were positive for viral agents detected in both genders. The most frequently detected viruses were enteroviruses 35/72 (48.6%) and herpesviruses 27/72 (48.6%). The other detected viruses included adenovirus, enterovirus, poliovirus types 1 and 2, poliovirus types 3 and 4, and WNV (Table 2). The participant age range was 0 - 14 years. Figure 1 shows the distribution of the most frequent viral meningoencephalitis among different age group.
This study analyzed 50 positive viral samples from Sheikh Hospital in Mashhad and 22 from Imam Reza Hospital in Bojnourd. Key findings include the exclusive detection of HSV (10 cases) and poliovirus types 3 and 4 in children hospitalized in Mashhad. Conversely, poliovirus types 1 and 2 were exclusively identified in children from Bojnourd Hospital. A significantly higher prevalence of viral meningoencephalitis attributed to enteroviruses (25 cases) and CMV (7 cases) was observed in Mashhad compared to Bojnourd (10 cases of enteroviruses and 3 cases of CMV). Additionally, adenovirus (3 cases) and VZV (7 cases) were detected solely in children hospitalized in Bojnourd, along with a single positive case of WNV.
Table 2 displays the prevalence rates, with enterovirus having the highest rate, followed by HSV, CMV, VZV, WNV, adenovirus, and poliovirus. Based on previous research and clinical significance, it is important to mention that the primers utilized in this project can detect HSV types 1 and 2, poliovirus types 1 and 2, poliovirus types 3 and 4, and enterovirus types 70 and 71.
According to Figure 1, the majority of enterovirus infections are found in children under age one. Herpesvirus and CMV infections follow closely behind. Notably, instances of poliovirus infection were solely observed in children over five years old. Interestingly, no cases of HSV or WNV were detected in children over five years old.
5. Discussion
The onset of viral meningitis is critical, as it signifies the virus's entry into the body via respiratory secretions or the fecal-oral route. This initial infection in the respiratory or gastrointestinal tract is followed by a secondary infection of the CNS, which can lead to severe conditions like meningitis or other neurological complications. The virus can infiltrate the CNS through various mechanisms, including infection of the choroid plexus epithelium and lymphoid tissue, initiation of inflammation and disruption of the blood-brain barrier, and infection of peripheral sensory neural pathways (7, 18). This study presents the frequency of nine viral pathogens linked to meningoencephalitis. Our findings revealed that enteroviruses had the highest prevalence, followed by HSV, CMV, VZV, WNV, adenovirus, and poliovirus, respectively. These results align with those observed in other countries, such as the United States (2), China (5), France (19), Spain (20), and India (21), where enteroviruses were commonly identified as a pathogen of viral meningitis.
In a global study conducted by Pormohammad et al. in 2022, it was found that enteroviruses are the most prevalent viruses in viral meningitis (22). A study in Yasuj, Iran, involving 104 patients, indicated that enteroviruses are the most common viral agents and that the mumps virus, HSV, and VZV are the endemic causes of viral meningitis in this region (23). In Isfahan, another city in Iran, a study involving 103 patients found the following distribution of viral causes: Enteroviruses (56.1%), HSV 1/2 (31.7%), Epstein-Barr virus (17.1%), VZV (9.7%), H1N1 influenza virus (4.9%), and the mumps virus (2.4%) (24). In research conducted by Sanaei Dashti et al. in Shiraz, Iran, in 2020, 56 patients were investigated, with only 21 (38.9%) testing PCR positive. The most common viruses found were enteroviruses (42.85%) and the mumps virus (38.1%), while VZV and measles were not found (25).
In Cambodia, the primary cause of meningoencephalitis was the Japanese encephalitis virus, with dengue virus as the secondary agent and enteroviruses as the third agent (26). A study conducted in China from 2007 to 2019 revealed that JEV was the leading encephalitis pathogen, followed by enteroviruses and the mumps virus (27). In Iran, enteroviruses emerged as a significant cause of meningitis, with a prevalence of 37.1% in neonates and 34.7% in children, making them a common cause (> 30%) of meningitis (28). Additionally, research by Modarres in Iran highlighted the mumps virus and enterovirus as significant causes of meningitis and meningoencephalitis among children (29). In southern Iran, Hosseininasab et al. found that non-polio human enterovirus and mumps virus prevalence was 43.3% and 36.7% among children aged 2 to 15 (30). Other viruses, such as arboviruses and lymphocytic choriomeningitis, rarely cause meningoencephalitis, and there have been relatively few reports of meningoencephalitis caused by COVID-19 (31).
The study focuses on a specific geographical area, utilizing samples only from three tertiary university hospitals. This limitation restricts the ability to generalize the findings to other regions or countries, as the prevalence of viral pathogens may vary significantly among different populations. Additionally, the research exclusively involves children under 14 who display symptoms of meningitis and encephalitis, thereby rendering the results inapplicable to adults or other age groups. Moreover, the study's cross-sectional nature provides only a snapshot of viral prevalence at a particular time, hindering the ability to establish cause-and-effect relationships or observe trends in infection rates over time. Using a convenience sample may also introduce bias, as the selected children might not accurately represent the broader population of children with meningoencephalitis in the region. Furthermore, the study screened for a more common viral pathogens, which raises the possibility that other viral or non-viral causes of meningoencephalitis were overlooked, leading to an incomplete understanding of the etiology of these conditions. While PCR was employed to identify viral pathogens — a sensitive method — the study does not provide details on specific viral loads, which could affect the clinical relevance of the findings. Lastly, although previous studies on meningoencephalitis in other regions of Iran and worldwide are discussed, the current study's results may be constrained by the absence of clear comparisons regarding research methodologies, timeframes, and geographical contexts. Furthermore, subsequent investigations should encompass an evaluation of clinical manifestations, incorporate patient age as a variable, and employ rigorous statistical methodologies to facilitate a more comprehensive understanding of this disease. Overall, the findings are primarily relevant to the main clinical settings located in two capital cities of northeastern Iran involving children under 14 with suspected meningoencephalitis, and further research is needed to extend these conclusions to other populations.
5.1. Conclusions
Our research indicates that viral agents were detected in more than 50% of the cases. The most frequently occurring viral pathogens were enteroviruses and herpesviruses. The noted regional and age-related distribution of viral agents, like enteroviruses in infants under one year old, along with the exclusive identification of some viruses in particular hospitals, emphasizes the necessity for geographically customized diagnostic and preventive approaches. Future studies should investigate the prevalence of viral meningoencephalitis in other age and reign groups.