1. Background
In December 2019, the first case of emerging acute respiratory syndrome due to coronavirus 2 was identified in Wuhan, China, and was named coronavirus disease 2019 (COVID-19). By March 2020, it had developed into a pandemic (1). Most children with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are asymptomatic or may have mild symptoms like fever, cough, and gastrointestinal symptoms; however, they can also experience severe forms of acute respiratory distress syndrome and death (2). A novel post-infectious hyperinflammatory syndrome known as multisystem inflammatory syndrome in children (MIS-C) was also described in pediatric patients (3). SARS-CoV-2 can lead to hematological abnormalities, including thrombotic events and anemia. Anemia and COVID-19 are a high-risk combination, as both conditions contribute to hypoxia and reduced oxygen availability. The hyperinflammatory state in COVID-19 alters iron homeostasis, characterized by increased iron consumption, reduced intestinal absorption, reduced erythropoiesis, and finally, anemia of inflammation (4). The relationship between anemia and COVID-19 is bidirectional: pre-existing anemia can exacerbate the severity of COVID-19. Hemoglobin (Hb) is an important determinant of the oxygen-carrying capacity; the presence of anemia may exacerbate peripheral tissue demand for oxygen in the context of COVID-19 (5). In May 2023, the WHO chief declared the end of COVID-19 as a public health emergency, but he also emphasized that it is still a global threat (6). Based on the real-time statistics website, there are more than 22,000,000 currently infected patients (March 2024) (7). Long-term complications of COVID-19 are also well described by several authors, highlighting the obscure and complex nature of this infection (8, 9). Pediatricians were confronted by significant changes in the epidemiology of childhood diseases and serious illnesses after the COVID-19 pandemic, which they had not seen before, including unexplained thrombotic events, ominous cases of varicella, and severe forms of empyema in children (10).
2. Objectives
This study aimed to investigate the relationship between COVID-19 infection and on-admission anemia in critically ill children, assessing their effects on outcomes. To our knowledge, this is the first study addressing this issue in pediatric patients.
3. Methods
3.1. Study Design and Participants
This retrospective, cross-sectional, single-center study was conducted at Children’s Medical Center, a public tertiary referral and academic hospital, and the Pediatrics Center of Excellence, Tehran, Iran. The research deputy and ethics committee of Tehran University of Medical Sciences approved it under the approval code IR.TUMS.CHMC.REC.1402.028. As this was a retrospective cross-sectional study with no need for medical intervention, the requirement for informed consent was waived. Patient information was anonymized before analysis. Several domestic outbreaks occurred in Iran from January 2020 to May 2023. During six peaks, we dedicated a separate ICU to patients, the COVID-19 PICU. During other periods, infected patients were admitted to isolated rooms in our regular PICUs. The data of this study belong to patients admitted to the COVID-19 PICU during outbreaks. We included all children hospitalized during the study period who met the inclusion criteria by using data from the hospital register. However, we conducted a post hoc power calculation to determine if the sample size was sufficient. The inclusion criteria included: (1) Admission to the COVID-19 PICU, AND (2) age 1 month to 18 years old, AND (3) at least one of the following: (1) Positive reverse transcription polymerase chain reaction (RT-PCR) nasopharyngeal swab for SARS-CoV-2, OR (2) clinical signs and symptoms consistent with COVID-19 diagnosis (fever, cough, respiratory distress, gastrointestinal symptoms, etc.) (11), OR (3) radiologic findings consistent with COVID-19 diagnosis (ground glass opacities and consolidations on chest X-rays or computed tomography (CT) scans that dominantly involve the periphery of bilateral lobes) (12, 13). We excluded any patient who was initially considered to have COVID-19 if the final diagnosis was otherwise. We also excluded known and new cases of hemoglobinopathies and patients with multisystem inflammatory syndrome in children (MIS-C).
3.2. Data Collection and Outcomes
A questionnaire was designed to record the patient’s characteristics, including demographic features, prehospital significant comorbidities, laboratory findings, and anthropometric data. Comorbidities were reported based on the International Classification of Diseases 11th revision, attributed to WHO (14). The data were extracted from electronic records of the hospital. The primary outcome was to evaluate the relationship between on-admission anemia and mortality in ICU-hospitalized children with COVID-19. For secondary outcomes, we studied the relationship between anemia and disease severity-related outcomes, including vasoactive need, respiratory support level, and ICU and hospital length of stay (LOS). The prognostic ability of ferritin levels was also studied. Data were collected using electronic files of patients. Patients were divided into two groups based on their first hemoglobin level upon hospital arrival as anemic and non-anemic. Reference ranges for hemoglobin and hematocrit levels in children vary with age and sex. For children aged 6 months to 18 years old, the threshold for defining anemia was hemoglobin at or below the 2.5th percentile for age and sex (15). For young infants aged 1 month to 6 months, values below -2SD were defined as anemia (16). To evaluate anthropometric status, we applied Centers for Disease Control and Prevention (CDC) recommendations for healthcare providers. We used WHO growth charts for infants and children aged 0 - 2 years and CDC growth charts for children aged 2 - 18 years (17). For statistical analysis, overweight and obesity were merged.
3.3. Statistical Analysis
Descriptive statistics, including mean (standard deviation) and frequency (percentage), were used to summarize patient characteristics. t-test and chi-square test were used to analyze differences between two groups. Univariable logistic regression was performed to assess the unadjusted association between anemia and mortality. Multivariable logistic regression was applied to adjust for potential confounders. Stata software version 14 was also used to find the best-fitted model. ROC curve analysis determined the optimal ferritin cut-off value for predicting mortality. All analyses were conducted using SPSS version 26. A P-value < 0.05 was considered statistically significant. In terms of information bias, we made efforts to consistently collect information. For laboratory results, all samples were tested in the hospital laboratory. In terms of selection bias, since the hospital serves as a referral center across the country, the patients may not be a good representation of the general population. This would be considered one of the limitations of our study. As mentioned earlier, we utilized multivariable methods to address potential confounders.
4. Results
4.1. Demographic Features, Anthropometric Status, and Laboratory Findings
We reviewed the medical files of 155 patients; out of them, 133 patients were enrolled, including 60 females (45.1%) and 73 males (54.9%). The mean age of patients was 63.2 months, and the median age was 48 months. Fifty-eight (43.6%) patients had anemia for age, and 75 (56.4%) were non-anemic. Among females, 53.5% were anemic, while only 35.6% of males were anemic. This was a significant difference (P = 0.04). The odds ratio (OR) was 2.06 (95% CI: 1.02 - 4.15), indicating that the odds of anemia are two times greater in females than in males (Table 1).
| Variables | All (N = 133) (100%) | Anemic (N = 58) (43.6%) | Non-anemic (N = 75) (56.4%) | P-Value |
|---|---|---|---|---|
| Age (mo); Mean | 63.2 | 58.8 | 66.6 | 0.44 |
| Sex | 0.04 | |||
| Male | 73 (54.9) | 26 (35.6) | 47 (64.4) | |
| female | 60 (45.1) | 32 (53.3) | 28 (46.7) | |
| Anthropometric data | 0.61 | |||
| Underweight | 66 (53.2) | 27 (40.9) | 39 (59.1) | |
| Normal | 32 (25.8) | 16 (50.0) | 16 (50.0) | |
| Overweight/obesity | 26 (21) | 10 (38.5) | 16 (61.5) | |
| Comorbidities | 0.03 | |||
| Previously healthy | 30 (22.6) | 6 (20.0) | 24 (80.0) | |
| Chronic disease | 103 (77.4) | 52 (50.5) | 51 (49.5) | |
| Case index | 0.89 | |||
| Positive | 55 (52.9) | 24 (43.6) | 31 (56.4) | |
| Negative | 49 (47.1) | 22 (44.9) | 27 (55.1) | |
| Clinical manifestation | ||||
| Fever | 95 (71.4) | 46 (48.4) | 49 (51.6) | 0.07 |
| Cough | 65 (48.9) | 27 (41.5) | 38 (58.5) | 0.63 |
| Constitutional symptoms | 28 (21.1) | 16 (57.1) | 12 (42.9) | 0.10 |
| Skin and mucus membrane | 5 (3.8) | 3 (60.0) | 2 (40.0) | 0.65 |
| Respiratory distress | 91 (68.4) | 40 (44.0) | 51 (56.0) | 0.90 |
| Gastrointestinal symptoms | 41 (30.8) | 19 (46.3) | 22 (53.7) | 0.67 |
| Sore throa | 4 (3.0) | 3 (75.0) | 1 (25.5) | 0.31 |
| Oxygen ≥ 90 | 22 (16.5) | 8 (36.4) | 14 (63.6) | 0.45 |
| Oxygen < 90 | 38 (28.6) | 12 (31.6) | 26 (68.4) | 0.07 |
| Neurologic symptoms | 20 (15.0) | 4 (20.0) | 16 (80.0) | 0.02 |
| Other | 27 (20.3) | 14 (51.9) | 13 (48.1) | 0.33 |
| Vasoactive agent | 0.14 | |||
| Yes | 73 (54.9) | 36 (49.3) | 37 (50.7) | |
| No | 60 (45.1) | 22 (36.7) | 38 (63.3) | |
| Respiratory support | 0.15 | |||
| Headbox/mask | 34 (27.4) | 16 (47.1) | 18 (52.9) | |
| NIV | 37 (29.8) | 12 (32.4) | 25 (67.6) | |
| Mechanical ventilation | 53 (42.7) | 28 (52.8) | 25 (47.2) | |
| PICU length of stay, day, mean | 6.9 | 7.7 | 6.3 | 0.32 |
| Hospital length of stay, day, mean | 15.7 | 18.5 | 13.5 | 0.07 |
Demographic Features of Patients and Secondary Outcomes
In our study, 30 (22.6%) patients were previously healthy, and 103 (77.4%) patients had at least one moderate to severe prehospital comorbidity. The most common underlying diseases based on ICD-11 were mental, behavioral, and neurodevelopmental disorders (25.2%), diseases of the circulatory system (16.3%), and neoplasms/diseases of the blood or blood-forming organs (14.8%) (see figure in appendix 1). Among healthy children, only 6 (20.0%) patients had anemia. This was a significant finding (OR: 0.24, 95% CI: 0.09, 0.65, P = 0.03), indicating that the odds of anemia in healthy children are less than in children with chronic disease. We also analyzed and compared the percentage of anemia in patients with underlying disease based on the disease category; we found no significant differences between them. The most common clinical manifestations were fever (71.4%), respiratory distress (68.4%), and cough (48.9%). Gastrointestinal symptoms (30.8%), oxygen saturation less than 90% (28.6%), and constitutional symptoms (21.1%) were also common. Neurological symptoms were found in 15% of patients (Table 1). Anthropometric data of 124 patients were available. Among them, 53.2% were underweight, 25.8% had normal weight, and 21% were overweight/obese. There was no significant difference between the two groups (P = 0.61) (Table 1). Eighty-seven patients had positive RT-PCR nasopharyngeal swabs for SARS-CoV-2, with 39% anemic and 61% non-anemic (P = 0.19). The laboratory data of patients were also studied and reported (Appendix 2).
4.2. Primary Outcome: Anemia and Mortality
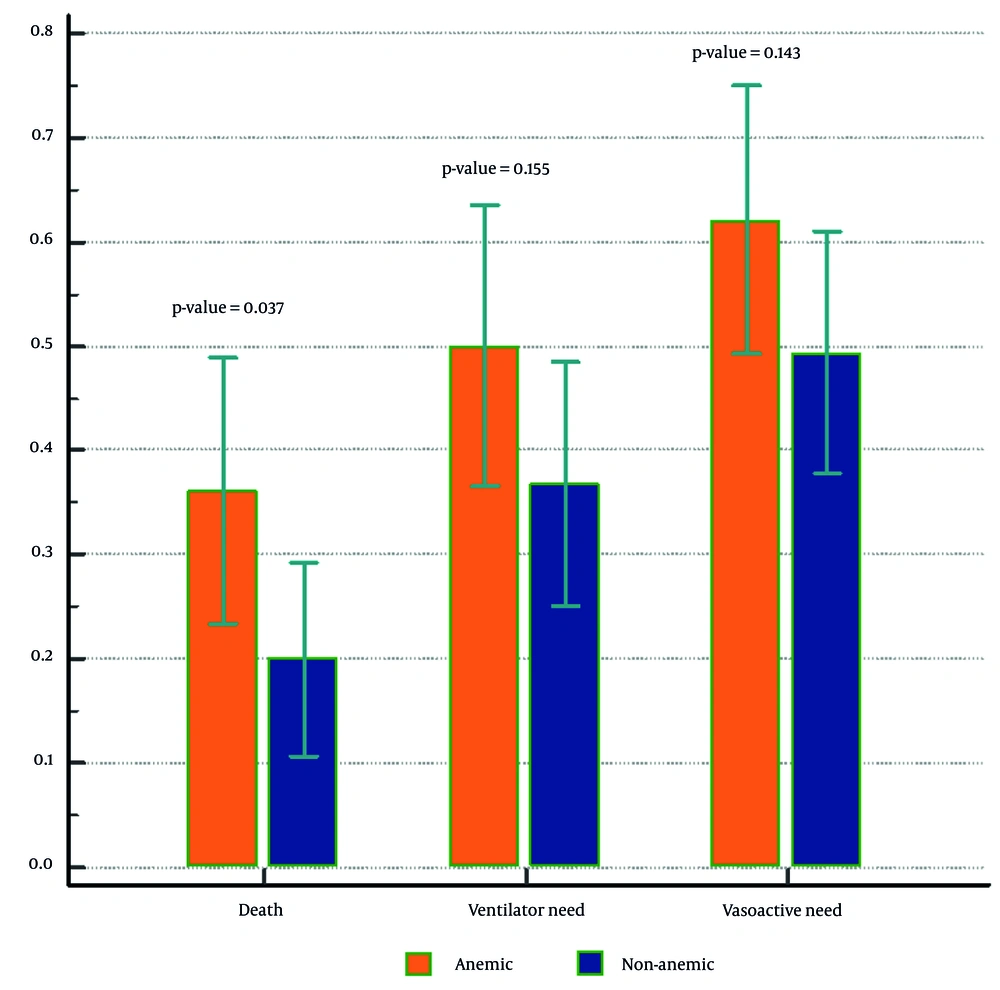
Ninety-seven (72.9%) patients survived, and 36 (27.1%) died. Among expired patients, 58.3% were anemic and 41.7% were non-anemic. This was a statistically significant finding (OR: 2.27, 95% CI: 1.04, 4.94, P = 0.03) (Table 2 and Figure 1). Multivariable logistic regression was used to adjust for comorbidity and sex. The OR for death in anemic versus non-anemic patients was 1.77 (95% CI: 0.79, 3.98, P = 0.16) adjusted for underlying disease and 1.82 (95% CI: 0.80, 4.12, P = 0.15) adjusted for both underlying disease and sex. Although these are not statistically significant and have wide confidence intervals, considering the OR, there is a small effect relationship between anemia and death (small effect size). Moreover, using the information criterion for comparing models, “adjusted model 1” was the best-fitted model (Table 2).
| Variables | Crude Model; OR (95% CI) | P-Value | Adjusted Model 1 b; OR (95% CI) | P-Value | Adjusted Model 2 c; OR (95% CI) | P-Value |
|---|---|---|---|---|---|---|
| Anemia | 0.039 | 0.162 | 0.152 | |||
| Yes | 2.27 (1.04, 4.94) | 1 (ref) | 1 (ref) | |||
| No | 1 (ref) | 1.77 (0.79, 3.98) | 1.82 (0.80, 4.12) | |||
| Blood transfusion | 0.00 | |||||
| Yes | 12.96 (4.85, 34.62) | |||||
| No | 1 | |||||
| Hb upon admission | 0.78 (0.67, 0.92) | 0.00 | ||||
| Hb on the final day in ICU | 0.71 (0.57, 0.88) | 0.00 |
The Logistic Regression Analysis of Anemia and Death as the Primary Outcome (Dependent Variable: Death) a
4.3. Secondary Outcomes
In total, 54.9% of patients needed vasoactive agents, among whom 49.3% were anemic. The proportion of patients based on the degree of respiratory support is also summarized in Table 1 (data from 124 patients were available). There was no significant difference between anemic and non-anemic patients in terms of vasoactive need and respiratory support level (P = 0.41 and 0.15, respectively) (Table 1 and Figure 1). The mean PICU and hospital length of stay (LOS) in all patients were 6.9 and 15.7 days, respectively. In anemic patients, these numbers were 7.7 and 18.5 days, respectively. In non-anemic patients, PICU and hospital LOS were shorter, at 6.3 and 13.5 days, respectively. However, the differences were not statistically significant (P = 0.07) (Table 1). We studied the rate of packed cell (PC) transfusions among patients. Among non-survivors, 83.3% received PC transfusions, compared to 27.8% of survivors. This was a significant finding (P = 0.00). According to univariate logistic regression, PC was used nearly 13 times more in non-survivors than in survivors (OR: 12.96, 95% CI: 4.85, 34.62) (Table 2). The mean hemoglobin (Hb) level on admission was 11.7 for survivors and 10.1 for non-survivors (P = 0.01). On the final day in the ICU, the Hb level was 10.9 in survived patients and 9.6 for expired patients (P = 0.00).
4.4. Ferritin Level and Disease Severity-Related Outcomes
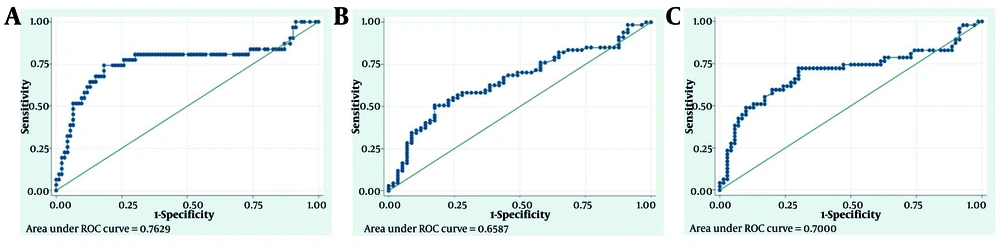
We drew the ROC curves of the ferritin level and disease severity-related outcomes and identified cut-off points. Figure 2 shows the details.
ROC Curves of ferritin level and disease severity-related outcomes. A, ROC Curves of ferritin level and death (empirical optimal cut point: 392, sensitivity at cut point: 0.74, specificity at cut point: 0.82, the area under ROC curve at cut point: 0.78); B, ROC Curve of ferritin level and vasoactive need (empirical optimal cut point: 330.5, sensitivity at cut point: 0.51, specificity at cut point: 0.82, the area under ROC curve at cut point: 0.67); C, ROC Curve of ferritin level and ventilator need (empirical optimal cut point: 206.5, sensitivity at cut point: 0.72, specificity at cut point: 0.70, the area under ROC curve at cut point: 0.71).
5. Discussion
In our study, 43.6% of patients were anemic upon admission. We found a significantly higher prevalence of anemia in females. In a multi-center study on children with SARS-CoV-2 in Oman, 38% of patients were anemic; anemia was also significantly more prevalent in children who required ICU admission (18). The prevalence of anemia is well-studied in adult patients with COVID-19 and is reported to range from 49.3% to 77% in ICU-admitted patients (19-21). This relatively wide range may be attributed to differences in demographics, comorbidities, and cut-offs used to define anemia. Saba et al. studied the prevalence of anemia among hospitalized children (22). They found that 72.79% of children were anemic, with a male-to-female ratio of 1.4:1. Our finding contrasts with other studies. It may be explained by nutritional, genetic, or environmental differences, which should be studied further. We showed that a remarkable number of patients had significant prehospital comorbidities, and anemia was significantly more common among them. This was expected, as anemia of chronic disease is among the most common causes of anemia in children (23). Iron deficiency anemia may also play a role in our patients with comorbidities. However, the type of anemia was not addressed in our study. In a multicenter study from Latin American countries on children with COVID-19, comorbidities were associated with an increased risk for hospital admission, ICU admission, and supplementary oxygen use (24). The distribution pattern of comorbidities in our region is almost similar to others (24, 25). The most common symptoms were fever, respiratory problems, and gastrointestinal symptoms, consistent with other studies (18, 24, 26, 27). Most of our participants were underweight. There was no significant difference between anemic and non-anemic patients regarding anthropometric data. Abdolsalehi et al. found that obesity was not associated with COVID-19 outcomes in children, but being underweight in the presence of comorbidities predicted a poor prognosis (28). In the study conducted in Latin American countries, 11.6% were underweight. The percentage of malnutrition was higher in our study. It may be explained by the high rate of comorbidities in our patients, which significantly affect nutritional indices (29). In our survey, 72.9% of patients survived, and 27.1% succumbed to COVID-19. Through univariate analysis, we found that death was significantly more common in the anemic group compared to children with normal Hb levels, with an OR of 2.27. We utilized logistic regression to adjust for potential confounders. This analysis revealed that death occurred 1.77 times more frequently in anemic patients versus non-anemic patients adjusted for comorbidity (95% CI: 0.79, 3.98, P-value: 0.162), and 1.82 times more often when adjusted for both comorbidity and sex (95% CI: 0.80, 4.12, P-value: 0.152) (Table 2). Despite the wide CIs and non-significant P-values indicating the low power of the study, mainly due to the small sample size, a small effect relationship between anemia and mortality is still evident in both analyses. Many studies considered anemia as a risk factor for mortality in adults with COVID-19 (30-32). Infancy, presence of comorbidities, severe illness, and mechanical ventilation (MV) were defined as predictors of mortality in pediatric populations (33). The overall mortality rate of our study was 27.1%. The mortality rate of COVID-19 was reported to be lower in other studies (25, 26). This can be explained by the fact that our survey was conducted on critically ill patients admitted to PICU. Mamishi et al. reported a mortality rate of 10% in our center in 2022, which shows that our hospital's overall mortality rate is close to other national and international reports (34). They also found that 93% of deaths occurred in our ICUs, which was a significant finding. Furthermore, our study was carried out in a tertiary referral hospital where more than three-quarters of patients had significant pre-hospital comorbidities, which is a major predictor of death among children with COVID-19. Variability in inclusion criteria, sample size, and ethnicity may also play a role in this finding. We found that more than half of the patients needed vasoactive agents, and half of them were anemic. 42.7% of children were intubated, while others were treated with non-invasive ventilation or supplemental oxygen. There was no remarkable difference between anemic and non-anemic patients considering vasoactive need and respiratory support level. Our result aligns with a survey done in Turkey in 2020 (21). In the study done in India, MV and hemodynamic support were needed in 28.3% and 37% of children with COVID-19, respectively (25). Although anemia decreases oxygen delivery to organs, adversely impacts the heart, and activates compensatory cardiovascular responses, particularly during critical illness, our result did not show an increase in hemodynamic and respiratory support (35, 36). It may be related to the severity of anemia, which was not studied. We also did not study the ventilator days and the inotropes scores in the survey, which will also affect the results. The mean PICU and hospital LOS were studied. These durations were shorter in patients with normal Hb levels compared to anemic children. However, the differences were not statistically significant. The studies done in Austria and Turkey support our findings (21, 30). We follow the TAXI protocol for PC transfusion in our division (37). We found more transfusion needs in children who succumbed to COVID-19. The first and final Hb levels of survivors were also significantly higher than non-survivors. Our results are close to Ozmen Suner et al’s findings (21). The mean of the first and highest ferritin levels in our study did not differ between patients with low and normal Hb levels. Literature has identified that elevated ferritin levels could be associated with adverse outcomes in patients with SARS-CoV-2 and serve as a severity and prognostic factor (38, 39). We found a cut-off point of 392 ng/mL for ferritin level, with 74% sensitivity and 82% specificity for predicting death. We also detected a cut-off point of 206.5 ng/mL for ferritin level, with 72% sensitivity and 70% specificity for predicting ventilator need. We also found that ferritin with a cut-off point of 330.5 ng/mL will predict vasoactive need, with 51% sensitivity and 82% specificity. Further study should be done to confirm our cutoff points. In a study done by Ombretta Para et al. in Italy on 200 adult patients with COVID-19, ferritin > 3000 ng/mL was correlated with unfavorable outcomes with a sensitivity of 34% and specificity of 96% (39). Considering this cutoff, 15 of our cases had ferritin > 3000 ng/mL. Among them, 80% required vasoactive agents, 87% underwent MV, and 73% expired. The present study has some limitations. The most important one is the small sample size. We did not address the severity or type of anemia (normocytic vs microcytic) or post-admission anemia. Additionally, we did not utilize severity scoring systems to predict PICU outcomes, such as the Pediatric Index of Mortality (PIM) or Pediatric Risk of Mortality (PRISM). We suggest that pediatricians screen for anemia and address it if needed, particularly in children with comorbidities, to potentially prevent severe outcomes associated with both anemia and severe COVID-19. Further prospective studies are encouraged to assess the relationship between anemia and severe outcomes of COVID-19 considering the type and severity of anemia.
5.1. Conclusions
In conclusion, the prevalence of on-admission anemia in children with COVID-19 in our PICU was 43.6%. The mortality rate in our study was 27.1%, and death was 2.27 times more likely in anemic patients compared to others. A majority of patients had comorbidities, and anemia was significantly more prevalent among them. Using logistic regression to adjust for comorbidity and sex, it showed that death occurred 1.82 times more often in anemic patients compared to non-anemic patients. Although this finding was not statistically significant, the odds ratio remains noteworthy and shows a small effect relationship between anemia and mortality.