1. Background
The incidence and mortality rates of lower respiratory tract infections in infants remain high (1), with acute bronchiolitis being one of the most concerning conditions. Acute bronchiolitis is a viral infection of the lower respiratory tract and is the most prevalent and primary cause of hospitalization in infants, especially those under two years old (2). Clinical manifestations vary from mild to severe, with potential progression to life-threatening respiratory failure. Current treatment guidelines emphasize supportive care, including hydration management and respiratory support, as specific therapies remain elusive. The use of salbutamol, corticosteroids, and antibiotics has not demonstrated clear benefits and may even pose risks (3). Because of the lack of effective specific therapies, reducing morbidity relies heavily on supportive care.
Airway edema and mucus plugging are key pathological features in acute bronchiolitis (4). Nebulizing 3% hypertonic saline (HS) solution may improve these pathological changes, but the evidence remains equivocal (5).
2. Objectives
This study aims to evaluate the efficacy of nebulized 3% hypertonic saline in improving clinical symptoms and reducing the length of hospital stay (LOS) in Vietnamese infants with acute bronchiolitis, thereby contributing to the evidence base for potential therapeutic interventions in this population.
3. Methods
3.1. Study Design and Participants
A randomized controlled open-label parallel-arm clinical trial, with an allocation ratio of 1:1, was conducted to investigate whether nebulizing 3% hypertonic saline improves clinical symptoms and reduces the LOS in infants with bronchiolitis compared with the control group. This study was conducted at the Respiratory Department of Can Tho Children’s Hospital, the central pediatric facility in Southwestern Vietnam, from August 2022 to May 2024.
3.2. Sample Size
The sample size for our study was calculated based on the mean hospital stay of infants with bronchiolitis treated with 3% hypertonic saline, which was reported to be 4.81 ± 2.14 days (6). With a power of 80% and a type I error rate of 5%, assuming a 20% reduction in hospital stay attributable to the intervention, the estimated sample size was 87 infants per group. Therefore, enrollment was planned for a total of 174 participants.
3.3. Inclusion and Exclusion Criteria
Pediatric patients were eligible for inclusion in the study if they were diagnosed with acute bronchiolitis from 1 to 24 months of age at Can Tho Children’s Hospital. Acute bronchiolitis is a diagnostic term that describes the clinical picture produced by multiple viral lower respiratory tract infections in infants and young children. They begin with upper respiratory symptoms such as coryza and cough with or without fever. After 24 - 48 hours, the respiratory findings observed in bronchiolitis include tachypnea, wheezing, hyperinflation of the chest, crackles, and rhonchi, which result from inflammation of the small airways (7-9). Diagnosis of acute bronchiolitis was performed directly by pediatricians.
Infants were excluded from the study if they met any of the following criteria: (A) Presence of underlying conditions such as congenital heart disease or chronic lung disease; (B) inadequate 3% hypertonic saline nebulization due to insufficient dosage, duration, or improper technique; (C) change in treatment from nebulizing 3% hypertonic saline to another type and vice versa; (D) severe respiratory failure requiring mechanical ventilation; (E) withdrawal of consent or self-discharge during the treatment period.
3.4. Randomization
Eligible infants were randomly assigned to one of two parallel groups (intervention and control), depending on whether the hospital admission was on an even or odd day. Those admitted on even days received standard medical care combined with nebulized 3% hypertonic saline. The others, admitted on odd days, were the control group, receiving standard medical care only.
3.4.1. Control Group
Children in the control group received only standard medical care, which included symptomatic treatment, oxygen therapy, and fluid management if needed (3).
3.4.2. Intervention Group
Children in the intervention group received standard medical care, along with nebulized 3% hypertonic saline to treat bronchiolitis. Each patient received 4 mL of 3% hypertonic saline via a firmly applied face mask, with an oxygen flow rate of 8 L/min, administered for 20 minutes at intervals of 8 hours. Nebulization continued until discharge. Infants receiving 3% hypertonic saline were closely monitored throughout the intervention and post-nebulization period. This continuous monitoring by a pediatrician allowed for prompt assessment and treatment of any adverse reactions. The majority of observed reactions were mild and self-limiting, resolving spontaneously during hospitalization without requiring specific intervention. In cases of bronchospasm, nebulized salbutamol was administered at a dose of 0.15 mg/kg/dose, with a minimum dose of 1.5 mg/time and a maximum dose of 5 mg/time. If a patient experienced oxygen desaturation, the nebulization session was stopped, and oxygen therapy was provided.
Therefore, our study was conducted exclusively in the inpatient respiratory department of the hospital.
3.5. Assessment and Data Collection
Infants with bronchiolitis were examined and assessed by the same pediatrician investigator to limit bias. Data were recorded using a standardized collection form, which included personal information (name, age, sex), medical history (e.g., underlying conditions, prior wheezing episodes, allergies), and clinical features (general condition, feeding and hydration status, respiratory rate, heart rate, temperature, oxygen saturation, wheezing, and use of accessory muscles). Clinical assessments were conducted at hospital admission (day 0) and at 24-hour (day 1), 48-hour (day 2), and 72-hour (day 3) intervals. Follow-up continued until discharge (10).
3.6. Operational Definitions
Operational definitions are as follows.
3.6.1. The Clinical Severity Score
This was scored according to clinical symptoms, including respiratory rate, wheezing, retractions, and general condition, on a scale from 0 to 3 points, with higher scores indicating more severity (Appendix 1 in Supplementary File) (11).
3.6.2. The Respiratory Distress Assessment Instrument
This focused on key markers of respiratory distress, specifically wheezing and retractions, with scores ranging from 0 to 17 points. Higher scores reflected more severe respiratory distress (Appendix 2 in Supplementary File) (12).
3.6.3. The Mean Length of Hospital Stay
This was defined as the number of days from admission to discharge for all participants.
3.7. Ethical Approval
The study was approved by the Ethics Committee in Biological Research at Can Tho University of Medicine and Pharmacy (No.173/PCT-HĐĐĐ, July 29th, 2022) and Can Tho Children’s Hospital. It was also registered on ClinicalTrials.gov (ID: NCT06558461). The parents or legal guardians of the participants were informed about the research and the safety of the intervention. The study received approval from all involved parties, including parents and caregivers of patients, through informed consent confirmation. Participants were assured that they could withdraw from the research at any time.
3.8. Statistical Analysis
Categorical variables were expressed as numbers and percentages, while continuous variables were summarized as either mean and standard deviation (SD) or median with minimum and maximum values, depending on the normality of the data distribution. The chi-square test or Fisher's exact test was used for categorical variables when the expected values in any contingency table cells were below 5. Comparisons of quantitative variables between the study groups at admission and during hospitalization were conducted using either the Independent Sample t-test or the Mann-Whitney U-test, depending on the normality of the distribution. Analyses were performed with version 26.0 of the statistical package for social sciences (SPSS) software (IBM Corp, Armonk, New York). All P-values are double-sided, with a P-value < 0.05 defined to indicate statistical significance.
4. Results
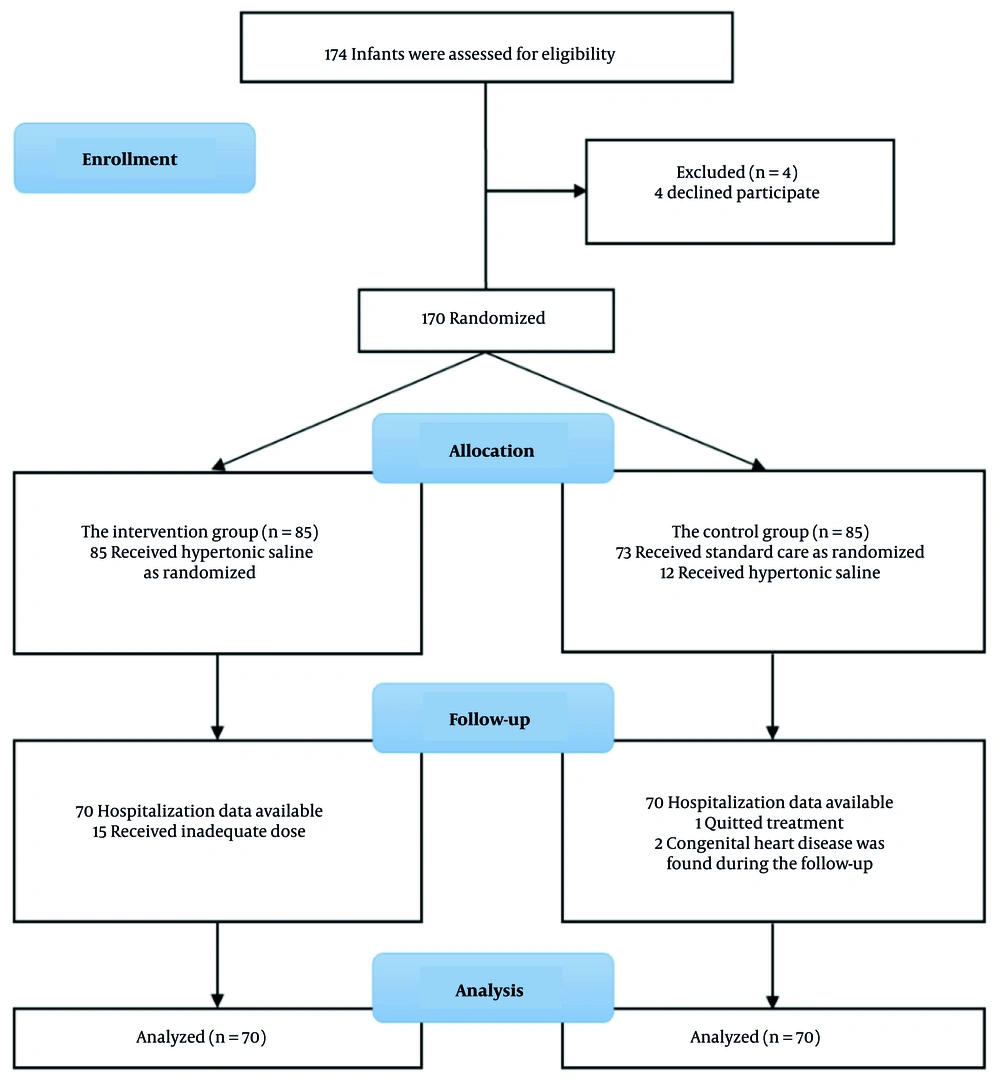
During the study period, 174 infants presenting with acute bronchiolitis at Can Tho Children's Hospital met the initial inclusion criteria. However, 34 infants were subsequently excluded due to underlying diseases, inadequate nebulization protocols, or withdrawal of consent. Ultimately, 140 patients were included in the final analysis, with 70 randomized to each of the intervention and control groups.
In the intervention group, we excluded 15 cases because these children did not receive the full 3 doses of 3% hypertonic saline per day during their hospital stay. There were several objective reasons, such as the child being absent for one or more nebulization sessions, or other doctors inadvertently administering nebulizers other than 3% hypertonic saline. In the control group, we excluded 12 children from the study because they received 3% hypertonic saline nebulization prescribed by other doctors who were unaware that these children were participating in our study. Additionally, we excluded one child because their guardian requested early discharge against medical advice. During the hospitalization and treatment period, we further excluded two children diagnosed with congenital heart disease (meeting our exclusion criteria) as discovered through echocardiography during their hospital stay (Figure 1).
The study groups were comparable at baseline, with no statistically significant differences noted in age, gender, respiratory rate, heart rate, oxygen saturation, clinical severity score (CSS), and RDAI score (P-value > 0.05 for all) (Table 1).
| Characteristics | 3% HS Group (n = 70) | Control Group (n = 70) | P-Value |
|---|---|---|---|
| Age (mo) | 5 (1 - 21) | 6.5 (1 - 18) | 0.086 b, c |
| Gender (male) | 39 (55.7) | 40 (57.1) | 0.671 d |
| Respiratory rate (min) | 54.4 ± 5.6 | 51.4 ± 5.8 | 0.828 e |
| Heart rate (min) | 132 (112 - 177) | 134 (110 - 166) | 0.497 b |
| Oxygen saturation (%) | 97 (93 - 99) | 97 (92 - 99) | 0.573 b |
| Reduced feeding | 45 (64.3) | 51 (72.9) | 0.267 d |
| CSS (point) | 5 (3 - 8) | 5 (2 - 8) | 0.274 b |
| RDAI (point) | 5 (2 - 10) | 5 (1 - 10) | 0.051 b, c |
Abbreviations: CSS, clinical severity score; RFAI, respiratory distress assessment instrument; HS, hypertonic saline.
a Values are expressed as median (min - max), mean ± SD or No. (%).
b Mann-Whitney U-test.
c P-value < 0.05 was considered statistically significant.
d Chi-square test.
e Independent sample t-test.
Regarding therapeutic outcomes, the intervention group demonstrated significant improvements compared to the control group. Notably, infants receiving 3% HS nebulization exhibited significantly lower CSS and RDAI scores throughout each day during the first three days of hospitalization (P < 0.001). The most pronounced effects were observed on the third day after admission. Furthermore, infants in the 3% HS group had a shorter hospital stay, with a median duration of 5 days (range: 3 - 10 days), compared to a median of 7 days (range: 3 - 15 days) in the control group. (Table 2)
| Variables | Characteristics 3% HS Group (n = 70) | Control Group (n = 70) | Z c | P-Value |
|---|---|---|---|---|
| CSS (point) | ||||
| Day 1 | 2 (0 - 5) | 3 (0 - 8) | -7.461 | < 0.001 |
| Day 2 | 3 (1 - 6) | 5 (2 - 8) | -9.388 | < 0.001 |
| Day 3 | 1 (0 - 3) | 2 (0 - 7) | -10.156 | < 0.001 |
| RDAI (point) | ||||
| Day 1 | 3 (0 - 6) | 4 (2 - 9) | -6.167 | < 0.001 |
| Day 2 | 2 (0 - 5) | 4 (2 - 8) | -8.923 | < 0.001 |
| Day 3 | 0 (0 - 3) | 4 (0 - 8) | -10.039 | < 0.001 |
| LOS (d) | 5 (3 - 10) | 7 (3 - 15) | -5.136 | < 0.001 |
Abbreviations: CSS, clinical severity score; RDAI, respiratory distress assessment instrument; LOS, length of stays; HS, hypertonic saline.
a Values are expressed as median (minimum - maximum).
b There were no adverse effects observed in children who used 3% HS in treatment.
c Mann-Whitney U-test with P-value < 0.05 was considered statistically significant.
5. Discussion
This randomized clinical trial involving 140 infants with acute bronchiolitis at the Can Tho Children’s Hospital demonstrated no significant differences in baseline characteristics, including CSS and RDAI scores, between the intervention and control groups at admission. However, after treatment with 3% HS nebulization, the intervention group exhibited significant improvements in both clinical severity and respiratory distress compared to the control group (P < 0.001). Moreover, the median LOS was significantly shorter in the 3% HS group (5 days) compared to the control group (7 days). Our findings regarding the median duration of hospitalization in the intervention group align closely with those reported in a recent review (6 days) (13), further supporting the potential benefits of 3% HS nebulization in reducing hospital stay for infants with acute bronchiolitis.
The findings of our study are consistent with the results of many previous research studies. A 2023 update to the previous systematic review by Zhang et al. included 34 randomized controlled trials (RCTs) with 5205 infants, of whom 2727 received HS, predominantly at a 3% concentration. This meta-analysis demonstrated that infants treated with HS had significantly lower CSSs compared to controls during the first three days of treatment. On day 1, data from ten trials (1 outpatient, 1 emergency department, and 8 inpatient trials) with 893 participants showed a mean difference (MD) of -0.64 (95% CI: -1.08 to -0.21). On day 2, the results from ten trials involving 907 infants indicated an MD of -1.07 (95% CI: -1.60 to -0.53), and on day 3, data from 785 infants in ten trials showed an MD of -0.89 (95% CI: -1.44 to -0.34). Moreover, nebulized HS was associated with a shorter mean hospital stay compared to standard treatment, with an MD of -0.40 days (95% CI: -0.69 to -0.11), based on 21 trials involving 2479 infants (5). Duration of hospital stay was defined as the time from hospital admission to discharge in all except two trials, which reported both time until fit for discharge and time until discharge (14, 15).
In line with our results regarding RDAI scores, Angoulvant et al. also reported a greater reduction in RDAI following 3% HS nebulization compared to the control group, with an adjusted difference of -0.7 (95% CI: -1.2 to -0.2) (16). These consistent results across multiple studies strengthen the evidence base for 3% HS as a valuable adjunct therapy in the management of acute bronchiolitis. In contrast to our findings, Jaquet-Pilloud et al. reported no significant difference in hospital stay duration between infants receiving 3% HS and those receiving standard care (MD: -0.12 days, 95% CI: -0.46 to 0.67) (17). This discrepancy might be attributed to variations in disease severity and population characteristics. Our trial enrolled 140 Vietnamese infants with acute bronchiolitis, whereas Jaquet-Pilloud et al. randomized 120 Swiss children with moderate to severe bronchiolitis, aged 6 weeks to 24 months. The difference in the severity of the condition at baseline may partly explain the variation in results. Our population had broader inclusion criteria, potentially including less severe cases, which may have allowed for a more pronounced response to the intervention with HS. Furthermore, our study demonstrated significant improvements in both CSS and RDAI scores after treatment with 3% HS, with these improvements sustained over the first three days of hospitalization. On the other hand, their study did not report significant clinical improvement between their intervention and control groups. These observations suggest that HS may be particularly beneficial in settings where a wider range of bronchiolitis severity is encountered, extending beyond just severe cases (17).
We found no adverse effects on the intervention members during the research periods. Most previous studies about 3% HS have also reported no side effects (6, 18-20). Some trials reported mild adverse events that resolved spontaneously (17, 21-23). Only one trial reported severe reactions, including desaturation and bradycardia, possibly related to hypertonic saline inhalation. However, this condition self-recovered within 24 hours (14).
One of the key strengths of this study is that it was conducted on hospitalized patients, allowing for more accurate and closer monitoring of symptoms compared to studies involving outpatients. Additionally, the research was conducted at the largest pediatric center in the Southwest region of Vietnam, and the study included a diverse patient population from various regions, not just a single city, enhancing the generalizability of the findings.
However, certain limitations need to be addressed. The study was primarily based on clinical assessment, which was affected by subjectivity, although potential bias was mitigated by employing a single investigator for symptom evaluation. Another limitation of our study is the even- and odd-day randomization, which does not ensure full allocation concealment. This method may lead to baseline imbalances if patient admissions vary by day. Although our analysis found no significant differences, selection bias cannot be ruled out. To further improve the robustness of future studies, we recommend implementing computer-generated randomization with sealed opaque envelopes or centralized randomization systems, which would ensure proper allocation concealment and eliminate any potential influence of admission patterns on group assignment.
Despite the improvements observed, not all infants responded equally, and the effect size, while meaningful, may not justify a universal recommendation without further validation in larger, multicenter trials. Future studies should explore long-term outcomes, cost-effectiveness, and patient-centered benefits to further solidify the role of 3% HS in bronchiolitis management. Additionally, while our study provides valuable insights into the efficacy of 3% hypertonic saline nebulization, not all infants responded equally. Multicenter trials and diverse patient populations are necessary to establish definitive conclusions and inform clinical practice guidelines.
5.1. Conclusions
In conclusion, our study demonstrates that nebulized 3% hypertonic saline is an effective and safe therapeutic intervention for infants hospitalized with acute bronchiolitis. It significantly improves both clinical severity and respiratory distress, as measured by the CSS and RDAI, respectively. Additionally, it leads to a reduction in the LOS. These findings support the use of 3% hypertonic saline nebulization as a valuable adjunct to standard supportive care in the management of acute bronchiolitis.