1. Background
Streptococcus pneumoniae is a leading cause of life-threatening infections in children, including pneumonia, meningitis, and bacteremia, accounting for an estimated 335,000 pediatric deaths annually (1). The increasing prevalence of antibiotic-resistant pneumococci has severely compromised traditional treatment strategies, with penicillin and macrolide resistance exceeding 40% in high-burden regions (2). This growing resistance complicates clinical management, leading to higher morbidity and mortality, particularly in low- and middle-income countries (LMICs) where access to appropriate antimicrobial therapy is often limited (3). Several factors contribute to the alarming rise of antibiotic resistance in pneumococci, including unregulated antibiotic use, overcrowded living conditions, and suboptimal pneumococcal vaccine coverage (4). Studies from Ethiopia and India report azithromycin resistance rates ranging from 50% to 60% (5, 6), while multidrug resistance (MDR) exceeds 30% in parts of Southeast Asia (7). These findings highlight the urgent need for targeted interventions, particularly in regions where pneumococcal surveillance remains inadequate. Iran faces similar challenges, with marked regional variability in pneumococcal resistance patterns. In Tehran, 43% of S. pneumoniae isolates are non-susceptible to penicillin, and clindamycin resistance exceeds 50% in unvaccinated populations (8). However, data from southern Iran — especially Bandar Abbas, a densely populated port city with high antibiotic consumption — remain scarce. Limited surveillance and underestimation of pediatric pneumococcal carriage rates further obscure the true burden of resistance in Pakistan (9). Iran confronts analogous challenges, characterized by regional disparities in resistance patterns. Local studies reveal significant deficiencies in surveillance, with pediatric carriage rates of S. pneumoniae likely underestimated and resistance profiles inadequately characterized (10). This study aims to bridge these gaps by analyzing the antibiotic resistance patterns and associated risk factors among pneumococcal isolates from children in Bandar Abbas. Monitoring asymptomatic carriers is critical for the early detection of emerging resistance, preventing transmission, and guiding public health interventions, even in the absence of symptomatic disease. By providing region-specific data, the findings can help refine treatment guidelines, inform public health policies, and reinforce the urgent need for expanded pneumococcal vaccination and antimicrobial stewardship in under-researched settings.
2. Objectives
This cross-sectional study aimed to determine the prevalence of S. pneumoniae carriage, assess antibiotic resistance patterns, and identify demographic, behavioral, and clinical risk factors linked to antibiotic resistance in children.
3. Methods
3.1. Study Design and Population
This cross-sectional study was conducted at Bandar Abbas Children’s Hospital, a prominent referral center in southern Iran, from October to December 2024. The study population comprised 390 children aged 6 months to 14 years who presented to the outpatient clinic for non-respiratory complaints. To minimize confounding factors, children with immunodeficiency, cystic fibrosis, malignancy, or recent hospitalization (within the past three months) were excluded.
3.2. Sample Collection and Microbiology
Nasopharyngeal swabs were collected using sterile cotton swabs. The swabs were immediately placed in Amies transport medium and transported to the microbiology laboratory within two hours for processing. Samples that required delayed processing were stored at 4°C for up to 24 hours. Isolates were identified through colony morphology, Gram staining, optochin susceptibility, and bile solubility. The swabs were streaked onto 5% sheep blood agar and incubated at 37°C in a 5% CO2 environment for 18 - 24 hours. Suspected S. pneumoniae colonies were identified based on morphological characteristics (alpha-hemolytic, mucoid colonies) and were confirmed through optochin susceptibility and bile solubility tests. Antibiotic susceptibility testing was conducted using the disk diffusion method, following CLSI/EUCAST guidelines, for nine antibiotics: Penicillin, amoxicillin, ceftriaxone, cefotaxime, azithromycin, clindamycin, rifampicin, vancomycin, and levofloxacin. The EUCAST standards guided azithromycin and clindamycin interpretations to align with European surveillance data, while CLSI guided β-lactams. The MDR was defined as non-susceptibility to three or more antimicrobial classes, as per WHO guidelines. Resistance patterns were categorized based on susceptibility testing results for the nine antibiotics tested. The S. pneumoniae ATCC 49619 was utilized as a control strain to ensure the accuracy of susceptibility testing.
3.3. Data Collection
A structured questionnaire was administered to parents or guardians to collect data on:
- Demographic factors: Age, gender, household size.
- Behavioral factors: Daycare/school attendance, exposure to household smoke (cigarettes or hookah).
- Clinical factors: Recent antibiotic use (within 1 and 3 months), history of hospitalization, recurrent otitis media, and pneumococcal vaccination status.
3.4. Statistical Analysis
Data were analyzed using SPSS version 27. Descriptive statistics (frequencies, percentages, means, and standard deviations) were used to summarize demographic and clinical characteristics. Associations between antibiotic resistance and risk factors were assessed using the chi-square test for categorical variables and Fisher’s exact test for small sample sizes. Multivariate logistic regression was employed to identify independent risk factors for resistance. A P-value < 0.05 was considered statistically significant.
3.5. Ethical Considerations
The study adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from parents or guardians, and ethical approval was granted by the Hormozgan University of Medical Sciences Ethics Committee (IR.HUMS.REC.1403.017).
4. Results
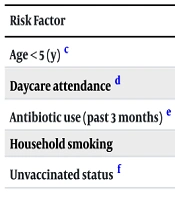
A total of 390 children were enrolled (Table 1), with S. pneumoniae isolated from 48 (12.3%) of nasopharyngeal swabs. Carriage rates varied significantly by age and risk factors (Table 2). Resistance rates were highest for clindamycin (28, 58.3%), azithromycin (22, 45.8%), and penicillin (20, 41.7%). Lower resistance was observed for ceftriaxone (10, 20.8%), vancomycin (10, 20.8%), and amoxicillin (8, 16.7%). No resistance to levofloxacin was detected. Vaccinated children (97, 24.9%) demonstrated marginally lower MDR rates (52.6% vs. 60.2% in unvaccinated children, P = 0.22). The MDR was observed in 58.3% of isolates (Tables 3 and 4). Significant associations were found between antibiotic resistance and age <5 years, daycare/school attendance, recent antibiotic use (within 3 months), history of hospitalization, and exposure to household smoke (Table 5). No significant links were found with gender, household size, or pneumococcal vaccination history.
| Variables | Frequency (%) |
|---|---|
| Age < 5 (y) | 180 (46.2) |
| Male gender | 210 (53.8) |
| Household size > 5 | 150 (38.5) |
| Daycare/school attendance | 90 (23.1) |
| Household smoke exposure | 72 (18.5) |
| Recent antibiotic use | 120 (30.8) |
| PCV10 vaccinated | 97 (24.9) |
| Factors and Categories | Carriage Rate (%) | P-Value a |
|---|---|---|
| Age group (y) | < 0.001 | |
| < 5 | 18.9 (n = 34/180) | |
| ≥ 5 | 6.7 (n = 14/210) | |
| Daycare attendance | 0.026 | |
| Yes | 25.6 (n = 23/90) | |
| No | 8.3 (n = 25/300) | |
| Household smoking | 0.048 | |
| Yes | 20.8 (n = 15/72) | |
| No | 10.1 (n = 33/318) |
a Statistically significant (P-value < 0.05), chi-square test used for comparisons.
| Antibiotic | Resistant | Intermediate | Susceptible |
|---|---|---|---|
| Penicillin | 20 (41.7) | 11 (22.9) | 17 (35.4) |
| Amoxicillin | 8 (16.7) | 7 (14.6) | 33 (68.7) |
| Ceftriaxone | 10 (20.8) | 6 (12.5) | 32 (66.7) |
| Cefotaxime | 18 (37.5) | 5 (10.4) | 25 (52.1) |
| Azithromycin | 22 (45.8) | 4 (8.3) | 22 (45.8) |
| Clindamycin | 28 (58.3) | 3 (6.3) | 17 (35.4) |
| Rifampicin | 20 (41.7) | 2 (4.2) | 26 (54.1) |
| Vancomycin | 10 (20.8) | 0 (0) | 38 (79.2) |
| Levofloxacin | 0 (0) | 0 (0) | 48 (100) |
a Values are expressed as No. (%).
| Resistance Profile | Frequency (%) |
|---|---|
| Penicillin + azithromycin + clindamycin | 10 (35.7) |
| Penicillin + cefotaxime + clindamycin | 8 (28.6) |
| Azithromycin + clindamycin + rifampicin | 6 (21.4) |
| Other combinations | 4 (14.3) |
a Chi-square test used for comparisons.
Abbreviation: aOR, adjusted odds ratios.
a Statistical tests: Multivariate logistic regression adjusted for covariates listed in Table 1.
b Statistically significant (P < 0.05).
c Reference: ≥ 5 years.
d Reference: No attendance.
e Reference: No recent use.
f Reference: Vaccinated.
5. Discussion
The high prevalence of MDR S. pneumoniae (58.3%) in Bandar Abbas presents a serious public health challenge, comparable to resistance rates reported in parts of Southeast Asia (30% - 60%) and sub-Saharan Africa (~ 50%) (5, 6). However, resistance rates for specific antibiotics vary significantly across regions. The observed penicillin resistance rate (41.7%) in Bandar Abbas is substantially higher than the 10% - 15% reported in Europe but aligns with findings from Tehran (43%) and Pakistan (38% - 45%) (8, 11). This suggests regional differences in antibiotic prescribing patterns and vaccine coverage. Similarly, ceftriaxone resistance (20.8%) in Bandar Abbas is notably higher than in Tehran (8% - 12%) and Pakistan (1%), indicating potential overuse of cephalosporins (8, 9). Macrolide resistance (azithromycin 45.8%, clindamycin 58.3%) is consistent with reports from India (50% - 60%) but exceeds resistance rates in Saudi Arabia (~ 30%) and Oman (~ 25%) (7). These findings highlight the need for antimicrobial stewardship programs to regulate antibiotic use and mitigate resistance.
Several key factors were significantly associated with increased resistance in this study. Recent antibiotic use (aOR: 4.5, P < 0.001), daycare attendance (aOR: 2.9, P = 0.004), and household smoke exposure (aOR: 2.4, P = 0.029), align with studies from India and Nigeria that have linked empirical antibiotic therapy to the emergence of MDR pneumococcal strains (6, 12). The widespread availability of over-the-counter antibiotics and incomplete treatment courses in Bandar Abbas likely exacerbate resistance. Strengthening antibiotic stewardship programs and enforcing stricter regulations on antibiotic sales could help curb this issue.
Children under five years old had a 3.2-fold higher likelihood of carrying resistant S. pneumoniae strains, consistent with studies from Brazil and South Africa (13, 14). This is likely due to their developing immune systems and increased exposure in daycare or preschool environments. In this study, daycare attendance was associated with nearly a threefold increase in resistance, underscoring the role of close-contact settings in bacterial transmission and antimicrobial selection pressure. Household smoke exposure significantly increased the risk of resistance (aOR: 2.4, P = 0.048), likely due to smoke-induced mucosal damage that facilitates bacterial colonization (15). Similar findings have been reported in other studies, emphasizing the need for public health initiatives to reduce indoor smoke exposure.
A particularly concerning finding was the low pneumococcal conjugate vaccine (PCV10) coverage (24.9%), far below the World Health Organization’s recommended 90% coverage. While vaccination status was not significantly associated with resistance (P = 0.089), inadequate vaccine uptake may contribute to the persistence of non-vaccine serotypes that are often resistant to antibiotics (8, 11). Expanding vaccine coverage is crucial for reducing pneumococcal carriage, transmission, and ultimately antibiotic resistance.
The resistance patterns observed in Bandar Abbas align with global trends but also reveal concerning regional variations. Ceftriaxone resistance (20.8%) was significantly higher than that reported in Tehran (8% - 12%) and Pakistan (1%), suggesting excessive cephalosporin use (8, 9). A study from the UAE (2010 - 2021) reported increasing resistance to levofloxacin, moxifloxacin, erythromycin, and trimethoprim/sulfamethoxazole (16). Similarly, in Oman, 56.8% of isolates were non-susceptible to at least one antibiotic, with 40.9% resistant to penicillin and 18.9% classified as multidrug-resistant (17). These findings emphasize the urgent need for targeted antimicrobial stewardship programs to optimize prescribing practices and limit resistance.
Addressing the increasing burden of MDR S. pneumoniae in Bandar Abbas requires a multifaceted approach:
- Strengthening antibiotic stewardship programs: Implementing evidence-based guidelines for antibiotic prescribing, promoting appropriate use, and restricting over-the-counter antibiotic sales are essential to curb resistance.
- Expanding pneumococcal vaccination coverage: Increasing PCV uptake could significantly reduce pneumococcal colonization and transmission, thereby lowering antibiotic resistance rates.
- Reducing household smoke exposure: Household smoke exposure significantly increased the risk of resistance (aOR: 2.4, P = 0.048), likely due to smoke-induced mucosal damage that facilitates bacterial colonization (15). Similar findings have been reported in other studies, emphasizing the need for public health initiatives to reduce indoor smoke exposure, particularly in households with young children or individuals with chronic respiratory conditions (18, 19).
- Enhancing surveillance systems: Robust surveillance programs should be established to monitor emerging resistance patterns, guide empirical treatment decisions, and inform vaccination policies.
- Molecular analysis of resistance mechanisms: Future research should incorporate whole-genome sequencing to track resistance gene evolution and assess the impact of PCV13 on serotype distribution.
5.1. Conclusions
This study highlights the alarming prevalence of MDR S. pneumoniae in Bandar Abbas and underscores the urgent need for targeted interventions. High resistance rates to penicillin, ceftriaxone, and macrolides, combined with low pneumococcal vaccination coverage and risk factors such as recent antibiotic use, young age, and household smoke exposure, present significant challenges for infection control. A collaborative effort between clinicians, policymakers, and public health authorities is essential to implement effective antimicrobial stewardship, expand vaccination programs, and enhance surveillance systems to combat the rising threat of pneumococcal resistance.
5.2. Limitations
- Single-center design: Findings may not be generalizable to other regions in Iran or globally due to localized data collection.
- Lack of molecular analysis: Absence of serotyping and resistance gene profiling (e.g., ermB, mefA) limits insights into resistance mechanisms and vaccine-serotype coverage.
- Small sample size of isolates: Only 48 isolates were analyzed, reducing statistical power and potentially missing significant associations.
- Cross-sectional design: Cannot establish causality between risk factors and resistance, only associations.
- Self-reported data: Risk of recall bias (e.g., antibiotic use) and underreporting (e.g., household smoke exposure).
- Low carriage rate (12.3%): Lower than global averages, possibly influenced by seasonal or demographic factors, raising questions about representativeness.
- Short study duration (3 months): Seasonal variations in pneumococcal carriage and antibiotic use were not accounted for.
- Vaccination coverage gaps: Low PCV10 uptake (24.9%) and lack of association with resistance may reflect confounding factors or insufficient data. Despite low PCV10 coverage, resistance rates were marginally lower in vaccinated children (52.6% MDR) compared to unvaccinated (60.2% MDR), though not statistically significant (P = 0.22).
5.3. Recommendations
- Expand surveillance systems: Implement multi-center, longitudinal studies across diverse regions to improve generalizability. Integrate molecular techniques (e.g., whole-genome sequencing) to track resistance genes and serotype distribution.
- Strengthen antimicrobial stewardship: Enforce stricter regulations on over-the-counter antibiotic sales. Educate healthcare providers on evidence-based prescribing and the public on antibiotic misuse risks.
- Boost vaccination coverage: Prioritize pneumococcal conjugate vaccine (PCV13) introduction and achieve WHO-recommended coverage (> 90%). Conduct serotype surveillance to align vaccination strategies with circulating strains.
- Public health interventions: Launch campaigns to reduce household smoke exposure (e.g., smoking cessation programs). Promote infection control measures in daycare/school settings to limit transmission.
- Policy and collaboration: Align national policies with global antimicrobial resistance (AMR) action plans (e.g., WHO’s global action plan). Foster partnerships between clinicians, policymakers, and international agencies to address AMR holistically.
5.4. Future Research
- Conduct longitudinal studies to assess temporal trends in resistance and vaccine impact.
- Investigate socioeconomic and cultural drivers of antibiotic misuse in the region.
- Future studies should incorporate serotyping and resistance gene profiling to inform vaccine strategies and resistance mechanisms.
- Multi-center collaboration: Larger, multi-center studies would enhance generalizability and statistical power.