1. Background
Acute gastroenteritis is an important cause of childhood morbidity and mortality, especially in children under 5 years old. Recently, it has been estimated that in developing countries, there are 450 million cases of diarrhea in children less than 5 years old annually and that 1-4% of them may die consequently. According to centers for disease control and prevention reports, Norovirus causes 23 million cases of acute gastroenteritis worldwide a year in all age groups (1). Norovirus can cause diarrhea in both adults and children (2). Noroviruses are most frequently recognized as a cause of gastrointestinal inflammation and causes more than 50% of all food borne disease in the US (3).
Among the viral infectious agents of acute gastroenteritis, Rotavirus, human Calicivirus, Astrovirus, and Adenovirus have been characterized (4, 5). The norwalk-like and sapporo-like viruses are recently renamed Norovirus and Sapoviruses, respectively. Norovirus is the one of the important triggers of febrile seizures (6). They are further divided into genogroups I and II, and which are responsible for approximately 179 million cases of acute gastroenteritis that occurs annually in the US (7). There are different methods to detect Norovirus such as ELISA (8), Real-Time (9), Rt-PCR (10), and cell culture (11). According to the varied etiology of Norovirus, they can be diagnosed by variety of diagnostic methods. In a study in Japan, the detection method of ELISA-kit is compared with RT-PCR . The ELISA method had a sensitivity of 76.3% and specificity of 94.9%. However because of the kit sensitivity to most Norovirus genotypes, it needs to be improved (8). In addition, according to a study on children under 12 years old with gastroenteritis during a 4 year period in Tunisia, it was shown that 64.8% were affected by Norovirus gastroenteritis (11).
2. Objectives
Considering the comparison of Norovirus diagnostic methods in different studies, we studied the prevalence of Norovirus which causes gastroenteritis in children under 5 years old suffering from diarrhea in 5 different cities of Iran by Rt-PCR in order to detect the number of positive samples.
3. Patients and Methods
In this study, 2170 samples of children with acute gastroenteritis, who were admitted to the pediatric hospitals in 5 cities of Iran (Tehran, Mashhad, Tabriz, Shiraz, Bandar Abbas) were collected. Written informed consent was obtained from all patients.
Fecal specimens were collected within 24 hours of admission. The specimens were frozen, sent to the laboratory, and subsequently stored at -70° C until Norovirus testing. Viral RNA was extracted from 30% stool suspensions in physiologic serum, then centrifuged at 6000 rpm for 20 minutes and filtered by 0.2 μm filter. Then 140 μL of the filtered samples was transferred to micro tubes and extracted by QIAamp viral RNA extraction mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The extracted samples were then stored at -20° C for a short time and further studied using Rt-PCR.
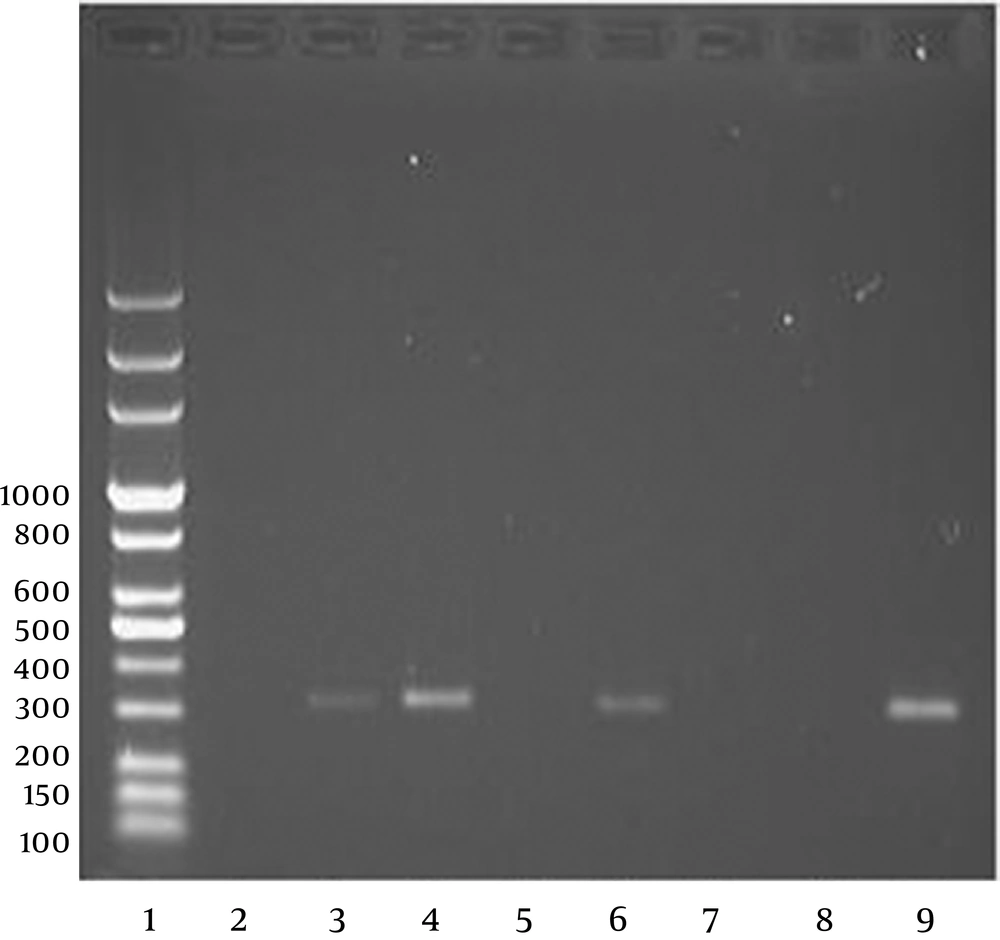
The cDNA synthesis was carried out using Rt-premix kit (Bioneer, U.S.A). The PCR products were analyzed by electrophoresis on a 1% agarose gel and visualized with UV light. TBE buffer was used as the electrophoresis gel (12). The PCR products obtained using the primer set JV12/ JV13 was 326 bp. The primer pair sequences used in the study has been shown in Table 1.
4. Results
Detection of Norovirus in 2,170 stool samples was done using Rt-PCR which was authenticated with approved primers for Norovirus (JV12/JV13). The prevalence of Norovirus in 5 large cities of Iran was reported to be 4.14%. There was no significant relationship between the patients` age and their Norovirus-caused gastroenteritis (P value < 0.05). The prevalence of Norovirus in different cities is studied and is shown in Table 2. Gel electrophoresis results are shown in Figure 1.
| City | Samples Positive | No. (%) |
|---|---|---|
| Tehran | 570 | 21 (0.97) |
| Tabriz | 360 | 14 (0.64) |
| Mashad | 240 | 4 (0.18) |
| Shiraz | 690 | 34 (1.50) |
| Bandarabbas | 310 | 17 (0.78) |
| Total | 2170 | 90 (4.14) |
5. Discussion
Noroviruses are one of the most important causes of acute non-bacterial gastroenteritis in children (1). This virus can be spread easily; however, there have been no reports of Norovirus prevalence in Iran during the last two decadesIn this study, Norovirus was responsible for 4.14% of all acute gastroenteritises and the prevalence rate varied in the 5 different cities. In another study in Iran by Romani et al. in which 4 of 93 (4.5%) samples were positive for Norovirus, 67 samples (61%) had been collected from inpatients and 26 (39%) from outpatients, all the positive samples belonged to the outpatients (13). In this study water resources for the patients were surveyed but no relationship between water and Norovirus infection was established. In countries with different dietary patterns, these statistics pattern may have some modifications. Norovirus outbreaks are reported to be 17.9% in Switzerland (14), 12% in Nicaragua (15), 21.9% in Taiwan (16), 9.9% in Pakistan (17), 11.9% in India (18), 48.4% in Italy (19), and 12% in Brazil (20). This virus causes about 40% to 50% of food-related diseases in the US (21). The high prevalence of gastroenteritis caused by Norovirus in these countries can be due to various factors different from the middle east. For example, the consumption of raw marine products such as oysters is one of the main causes of Norovirus spread in developed countries (22); however, this dietary pattern is rare in middle eastern countries (including Iran) (13). Therefore, the prevalence of Norovirus in these countries is less. Norovirus gastroenteritis is seen in all age groups and therefore there are not any age limitations for contracting of the virus (12). Although the risk of Norovirus gastroenteritis is all year-round, nevertheless the peak of Norovirus infection is in cold seasons (23) therefore this disease is frequently referred to as cold season diarrhea (24). Since Norovirus is an RNA virus, RNA instability in high temperatures can cause more outbreaks in the second half of the year. In this study, the relation between geographic distribution and Norovirus infection was also considered.
It was shown that patients referred to Shiraz hospital had a higher percentage of Norovirus infection and which would indicate a need to do a survey of Norovirus outbreak in this area.
To sum up, Norovirus should be considered as one of the prevalent causes of acute gastroenteritis in children less than 5 years of age in Iran as well as many other countries, however comprehensive research is needed to estimate the exact number of infected children using accurate molecular methods.