1. Introduction
Systemic mycoses are increasing in importance as opportunistic infections in various immune-deficient conditions. The underlying etiology of the microorganisms varies depending upon the type of immune dysfunction, and this often makes a definitive diagnosis difficult to establish.
Cryptococcosis was first described by Busse in 1894, and it is an uncommon systemic mycosis caused by the encapsulated yeast Cryptococcus neoformans. Approximately 85% of patients with cryptococcosis have impaired cell mediated immunity. Acquired immunodeficiency syndrome (AIDS) associated cryptococcal infections now account for 80-90% of all patients with cryptococcosis (1). Other causes of acquired immunodeficiency, such as immunosuppressive drugs, or congenital immunodeficiency, for example idiopathic CD4 T-cell lymphocytopenia, may also be present with cryptococcal infections. Here, our index case, a known case of focal segmental glomerulosclerosis (FSGS), who was taking various immunosuppressive drugs, including corticosteroids and mycophenolate mofetil (MMF) for the previous two years, presented with features of disseminated cryptococcosis.
2. Case Presentation
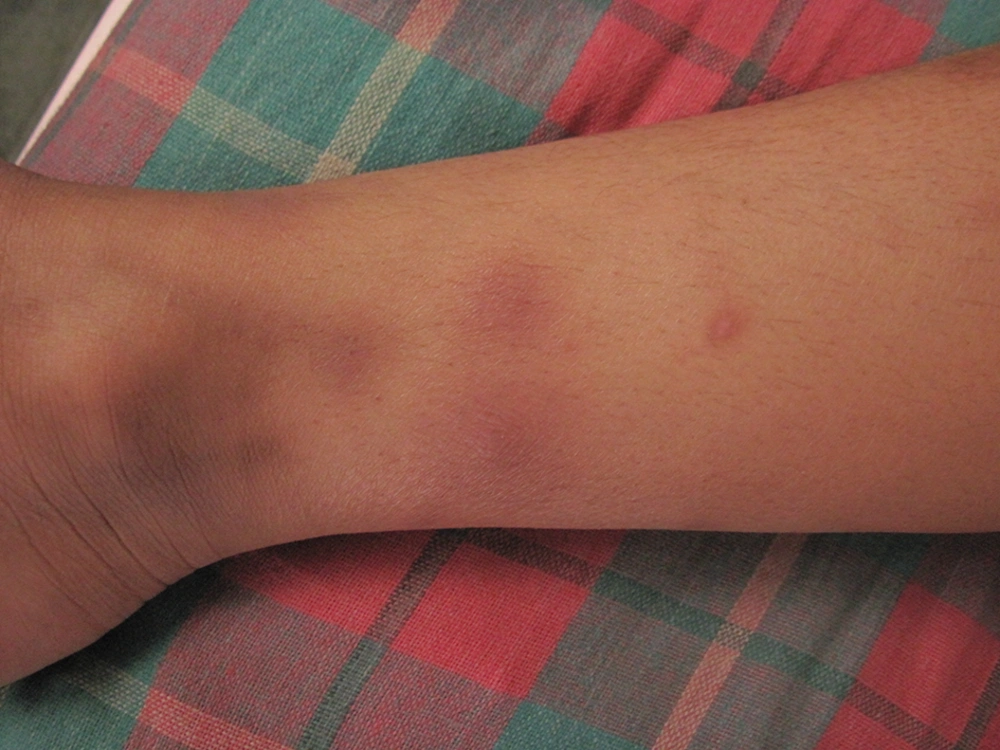
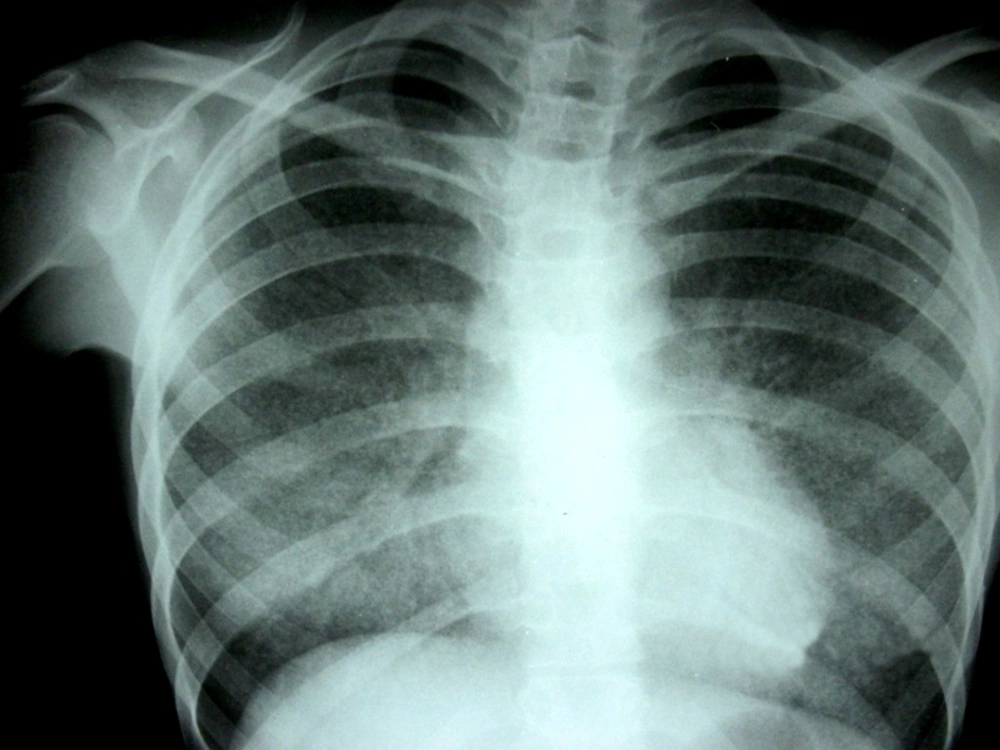
A 15 year old girl, with steroid resistant nephrotic syndrome (SRNS), secondary to FSGS, was admitted in 2010 in our hospital with a history of fever which had been present for a period of one month, and associated with a cough. She had initially been on prednisolone and cyclophosphamide, but as her nephrotic syndrome (NS) did not respond to treatment, she was started on mycophenolate mofetil (MMF) at 1200 mg/m2/day along with a low steroid dose. Then her MMF dose was increased further to 1500 mg/m2/day, as she continued to have nephrotic range proteinuria, and the area under the curve for mycophenolic acid was thought to be low. On admission, the girl was very sick and pale, in addition she was tachypneic and febrile. Crepitations were audible in her chest and she had multiple erythematous plaques (Figure 1) all over her body. These lesions were reddish, non-tender, non-pruritic, slightly raised, and 1-4 cm in diameter. Although her C–reactive protein was elevated (123 mg/L), her total leukocyte count was 11500/cmm, with 63% neutrophils. Liver function was normal, but there was significant renal impairment with a creatinine level of 2.3 mg/dL. A chest x-ray showed bilateral diffuse infiltration with miliary mottling (Figure 2). Computed tomography of the chest also revealed areas of patchy atelectasis, with consolidations having a ground glass appearance. Skin biopsy from the erythematous lesions was non-contributory. The MMF was stopped and she was started on a stress dose of steroids, along with antibiotics (co-amoxiclav and ofloxacin). Subsequently, as there had been no improvement, co-trimoxazole and azithromycin were added, keeping Pneumocystis carinii and Mycoplasma pneumonia in mind. Her symptoms failed to respond to any of these medications and there was a steady deterioration in her clinical status. After seven days we received the report of the BACTEC blood culture, which revealed the growth of Cryptococcus neoformans.
A lumbar puncture test was conducted. Cerebrospinal fluid (CSF) revealed 230 cells which were predominantly lymphocytic, the gram stain was negative, but it showed growth of a yeast like fungus. HIV serology was negative. On the basis of these reports, liposomal amphotericin B was started at 1.5 mg/kg/day. In the meantime, a culture sensitivity report of both blood and CSF revealed the fungi to be sensitive to fluconazole, ketoconazole, flucytosine and amphotericin B. The girl became afebrile on day eight of the amphotericin and was ultimately discharged on oral flucytosine and fluconazole, after receiving amphotericin for 21 days. On follow-up she has remained afebrile, her respiratory symptoms including cough, have improved, the skin lesions disappeared, and flucytosine was stopped after three months. The girl was on continuous ambulatory peritoneal dialysis for one year, and she has received a low dose steroid and regular follow-up for the past two years. Fluconazole will be continued until the patient remains immunosuppressed.
3. Discussion
Cryptococcosus neoformans has a worldwide distribution. Of the 19 species that comprise the genus Cryptococcus, human disease is associated only with Cryptococcus neoformans and Cryptococcus gattii (2, 3). The virulence of the organism plays a relatively minor role in determining the outcome of an infection; the crucial factor is the immune status of the host, especially in patients with acquired defective cell mediated immunity, such as patients with AIDS (1). Other major predisposing conditions include lymphoproliferative disorders, primary immunodeficiencies affecting both T- and B-cell lineages, and immunosuppressive therapies, including corticosteroids. Prolonged use of corticosteroids and other immunosuppressive agents for SRNS, such as FSGS, predisposes patients to develop significant infections with opportunistic organisms like C. neoformans. Patients with NS are susceptible to a variety of infections due to the numerous of changes that occur in the immune system, even in the absence of immunosuppressive treatment. Therefore, when they are put on immunosuppressive therapy they become even more susceptible to opportunistic infections. Although there are case reports of adults with nephrotic syndrome developing disseminated cryptococcal infections, a Medline search did not reveal any case reports of pediatric nephrotic syndrome on MMF developing a disseminated cryptococcal infection (4). MMF is a potent anti-proliferative agent and it is likely to enhance immunosuppression among nephrotic children.
C. neoformans usually evokes a granulomatous inflammation in immunologically competent subjects with some degree of variation in its pattern. However, patients with an altered immunologic mechanism, due to massive immunosuppressive chemotherapy and long treatment courses of corticosteroids, appear to fail in the formation of a granulomatous response and usually end up with disseminated life threatening infections. Duperval et al. observed systemic dissemination of cryptococcal infection in 59% of patients who had a cryptococcal infection and corticosteroid therapy (5). The principal site of cryptococcal infections include; pulmonary, central nervous system (CNS), skin and disseminated diseases. Disseminated cryptococcosis is defined as the recovery of Cryptococcosis neoformans from blood, sterile body fluids or tissues other than pulmonary tissue. Skin manifestations occur in 10% to 15% of cases and were one of the features of disseminated cryptococcosis even in our patient (6). Most of the time, disseminated infection follows a primary pulmonary disease, especially among immunocompromised persons.
Recovery of the fungus by culture from body fluids (like blood, CSF), or demonstration of the fungus in histologic sections of infected tissue, is definitive for a diagnosis of disseminated Cryptococcosis. A latex agglutination test, which detects cryptococcal antigen in serum and CSF, is the most useful diagnostic test. Titers of > 1:4 in bodily fluids are strongly suggestive of infection, and high titers of > 1:1024 reflect high burdens of yeast, poor host immune response, and a greater likelihood of therapeutic failure (7).
In general immunocompromised patients with disseminated cryptococcosis are recommended; induction of amphotericin B (0.7–1 mg/kg/day), plus flucytosine (100 mg/kg/day in 4 divided doses), for a minimum of two weeks. Depending on the clinical response, induction therapy may be continued for as long as 6–10 weeks. Induction is followed by a consolidation phase with flucytosine plus fluconazole, or itraconazole for 6 to 10 weeks, followed by prolonged maintenance on fluconazole or itraconazole (3, 8, 9). Lipid-complex amphotericin B (3–6 mg/kg/day) is recommended for individuals who are intolerant of desoxycholate amphotericin or have compromised renal function, like our index case.
Apart from requiring prolonged intensive therapy the mortality in cases of disseminated Cryptococcal infection can be as high as 15% to 30%. MMF is increasingly being used in children (10) with complicated nephrotic syndrome (steroid resistant/steroid dependent/frequent relapsers), but in view of our present case report, a high index of suspicion for disseminated Cryptococcus needs to be maintained in such children. In addition, the pediatrician should be cautious before increasing the dose of MMF beyond prescribed limits among non-responders.