1. Background
Acute respiratory tract infections (ARTI) are regarded as important causes of morbidity and mortality in pediatric patients. This refers to both outpatient visits and 20-40% of hospitalized patients (1, 2). The significant impact of these infections on developing countries is based on the fact that nearly 50% of pediatric consultations are related to the respiratory tract infection (RTI) (3). The world health organization (WHO) ranks the lower RTIs as the leading cause of disease worldwide, which accounts for 4 million deaths per year (2, 4). RTI can be life-threatening depending on the causative agent and host condition. A high incidence of RTI in children is due to increasing exposure of young children to siblings and friends with RTI, childcare centers, and environmental factors. Additionally, susceptibility to ARTI may be due to inherited disorders of the immune system, which are responsible for 45-60% of all acute respiratory diseases in infants and young children (2, 3). Most of these infections are caused by respiratory syncytial virus (RSV), influenza virus A or B (FluV), parainfluenza virus (PIV), rhinovirus (RV), and human adenovirus (HAdV). Several recently discovered viruses such as human metapneumovirus (HMPV), human bocavirus (HBoV), and human coronaviruses (HCoV) have been identified as potential respiratory pathogens (5).
Adenoviruses, first isolated in the 1950s from explanted adenoid tissue, are double-stranded non-enveloped DNA viruses that naturally infect many vertebrates including human and non-human primates (6). Human adenoviruses (HAdV) are grouped into seven species of A to G and to date, 56 different types (HAdV-1 to HAdV-56) have been described based on serology, whole genome sequencing, or phylogenomics (7-9). Adenovirus accounts for 5-15% of upper and lower RTIs in infants and children hospitalized for respiratory disease (2, 10). Clinical signs of an AdV infection are rather nonspecific and variable and include tonsillopharyngitis, conjunctivitis, pneumonia, gastroenteritis, hepatitis, and hemorrhagic cystitis (2). Fever, pharyngitis, tonsillitis, cough, and sore throat are the most common symptoms in affected children and young adults with AdV-associated RTI (8).
2. Objectives
According to the importance of the main detection of exact reason of respiratory infection in human being and though the adenovirus symptoms are like other viral infections, especially Influenza, we aimed to determine the prevalence of adenovirus infection in pediatric patients with respiratory symptoms.
3. Patients and Methods
3.1. Clinical Specimens
The specimens used in this study were from throat swabs from 328 pediatric patients with RTI admitted to the hospitals affiliated with Shiraz University of Medical Sciences from 2010 to 2012. The collected specimens were submitted to the influenza research center laboratory. The relevant clinical information was obtained through a standard questionnaire that itemized hospitalization status, age, sex, and clinical symptoms such as fever, cough, sneeze, and muscle ache. Throat swabs immersed in phosphate-buffered saline (PBS pH 7.2) and stored at -70℃ for subsequent DNA extraction.
3.2. Nucleic Acid Purification and PCR Screening
Nucleic acids from respiratory specimens were extracted using the DNA kit (AccuPrep®, Bioneer Corporation, USA) according to the manufacturer’s instructions. Human adenovirus extracted DNA was detected by nested-PCR of the hexon gene as described previously (11). The first round amplification was done by the forward primer 5´-GCC GAG AAG GGC GTG CGC AGG T <A>-3´ and the reverse primer 5´-TAC GCC AAC TCC GCC CAC GCG C <T>-3´. The second round amplification was performed by the forward primer 5´-TGA CTT TTG AGG TGG ATC CAT G <G>-3´ and the reverse primer 5´-GGT CTC GAT GAC GCC GCG GTG <C>-3, both 'targeting a portion of the HAdV hexon gene including 161 bp and 107 bp amplicons. PCR amplication was performed using 50 µL reaction volumes containing 45 µL mixture containing 1 mM Mgcl2, 5 µL PCR buffer 10 x, 0.1 mM each of deoxynucleotide triphosphate, 0.4 pmol/µL of each primer, 1 U of Taq DNA polymerase, and 5 µL of nucleic acid extract. The procedure included denaturation by one cycle at 94℃ for 10 min, followed by 33 cycles at 94℃ for 1 min, 61 for 1 min, 72 for 1 minute, and a final extension at 72℃ for 10 minutes. As for the nested-PCR reaction, 5 µL of the first-round PCR product was amplified as described above. PCR products were detected by 1.5% agarose gel electrophoresis followed by staining with ethidium bromide.
4. Results
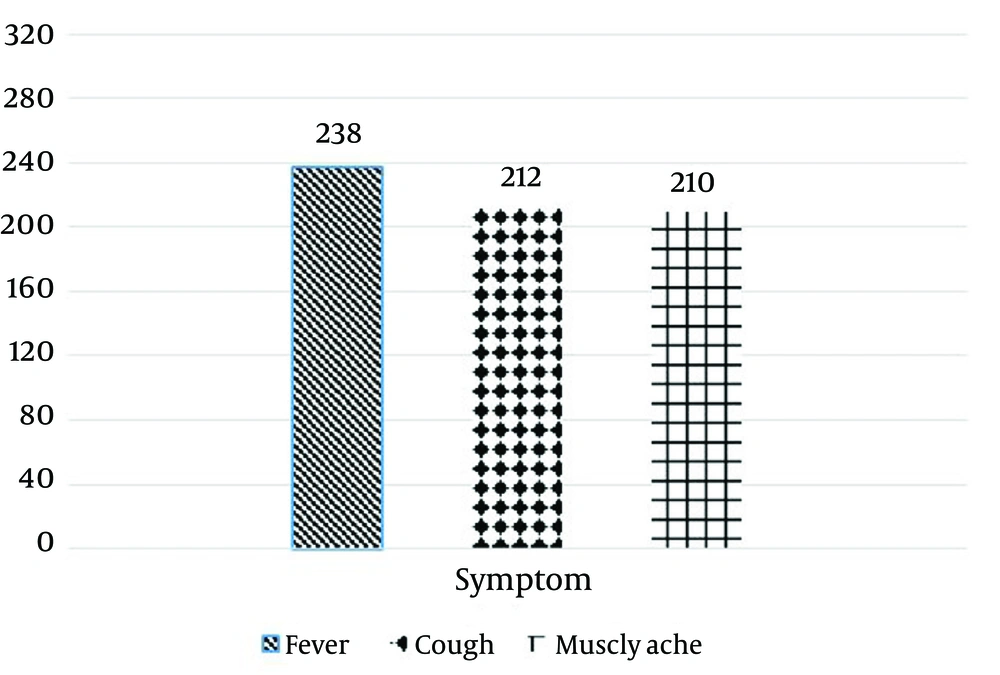
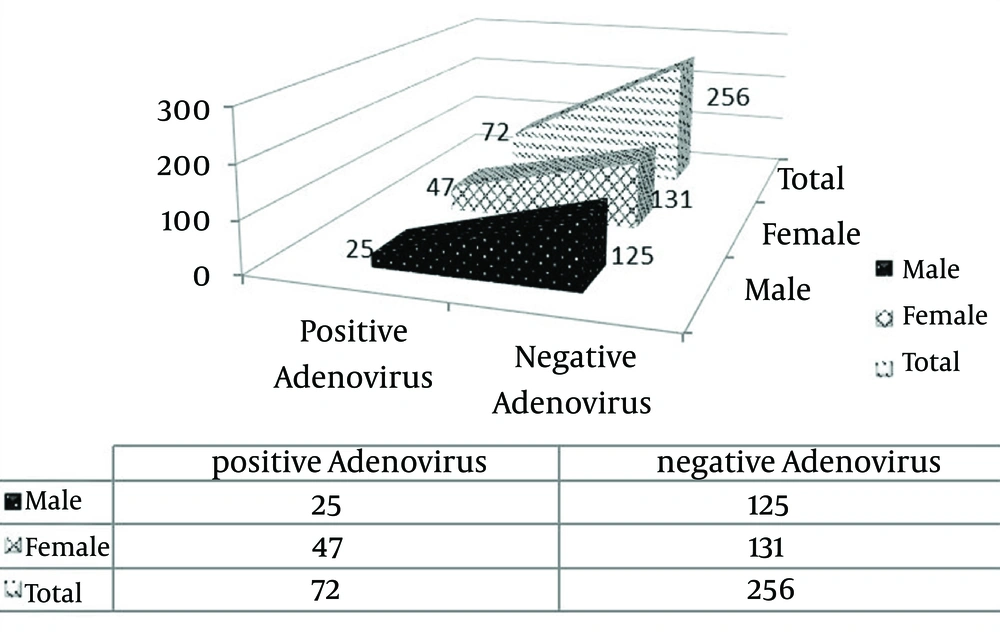
A total of 328 throat swabs were processed and the corresponding data are shown in Table 1. Only samples from patients younger than 19 years of age were included and among them, 192 (58.54%) and 136 (41.46%) samples belonged to the patients younger and older than five years of age, respectively. Additionally, 150 (45.73%) and 178 (54.27%) samples were collected from males and females, respectively. Amongst 243 (74.08%) patients hospitalized with ARTI, symptoms associated with fever, cough, and muscle ache were seen in 238 (72.56%), 212 (64.63%), and 210 (64.02%) patients, respectively (Figure 1). According to the nested-PCR results, the adenovirus was detected in 72 (21.95%) patients; the positive results were reported in 25 (34.72%) and 47 (65.27%) of males’ and females’ samples, respectively (Figures 2 and 3).
| Symptom | Frequency | Present Frequency, % |
|---|---|---|
| Fever | 70 | 97.22 |
| Cough | 63 | 87.44 |
| Muscle ache | 42 | 58.30 |
Fever was seen in 70 (97.22%) samples with positive results. Total analyzing results showed that most of the positive resulted for adenovirus were detected in samples from children older than five years of age. The majority of patients presented with upper respiratory tract symptoms including cough and muscular ache (Table 1), which are the most reported symptoms in other respiratory viral infections. According to the analysis of the results with SPSS software, we could not find any significant association between AdV positive samples and the age and gender of the patients; however, there was an association between seasons and epidemiology of the HAdV in the south region of Iran.
5. Discussion
Respiratory infection is a leading cause of morbidity, hospitalization, and death in pediatric patients (12). Approximately 80% of upper RTIs have viral etiology and can lead to asthma exacerbation and acute otitis media (13). Additionally, lower RTIs are manifested as bronchitis, bronchiolitis, and pneumonia (14). Adenoviruses are most important respiratory pathogens, and constitute 5-8% of all RTIs in infants and children younger than two years of age. HAdV infections are clinically similar to those of other respiratory viruses and the definitive determination of viral type and rapid diagnosis is problematic and cannot be achieved on the clinical ground. Due to the persistence and prolonged shedding of adenovirus, diagnostic approaches must be sufficiently sensitive to detect low levels of virus in clinical specimens. Adenovirus infections can be diagnosed by a variety of traditional and molecular methods. Molecular methods are most sensitive for detecting adenovirus in clinical specimens. The present study was conducted to detect HAdV by nested PCR. The frequency of HAdV in our study was 22% (72 out of 328 samples) that is slightly higher than the frequency of 14.4% reported by another study from Iran (15, 16), and several parts of the world including Oman (15%), Kenya (14%), Brazil (10%), Korea (10.3%), Australia (7.3%), Hung Kong (5.3%), Mejia (5.2%), and China (4.9%) (11, 17-22). According to these studies, HAdV is widespread throughout the world and is one of the causative agents of upper and lower RTIs in pediatrics. Amongst 72 cases with HAdV infections, 25 patients were males and 47 females. In contrary to other reports, female patients composed a higher proportion in our study (1, 2, 11, 18, 23).
Similar studies conducted by Arabzadeh et al. in Iran (24) and by Zou et al. in China showed no significant differences in the prevalence of HAdV infections between males and females. Adenovirus diseases usually result from reinfection and the elderly often have partial immunity due to pre-existing systemic and mucosal antibodies (25). In addition, elderly might have lesser amount of respiratory secretions and lower viral load in comparison to the children. Nearly 74% of children infected with HAdV were older than five years of age. However, other reports showed that HAdV mostly occur in children younger than two years of age, which is not in agreement with similar investigations conducted by Saffar et al. (2, 23, 26), Naghipour et al. and other reports across the world (27).
HAdV infections showed a variety of clinical symptoms. Most common symptoms observed in our study of 72 children with HAdV infections without co-infection were cough (64.63%), fever (72.56%), and muscular ache (64.02%). There is no specific symptom associated with HAdV infection and HAdV infected children reported by Shike et al. and Kahbazi et al. (16) exhibited non-specific symptoms such as fever and cough (10). The results of this study showed that acute respiratory adenovirus infection in Shiraz occurred mostly during months of November 2011 to February 2012. In contrast to other studies, HAdV was more prevalent in children older than five years of age. Additionally, the infection rate in females was higher than in males.