1. Context
Cytokines and chemokines are a group of small proteins that are critical to the maturation, differentiation, and activation of cells. They are also involved in cell mobility and consequently affect the integrity of the extracellular matrix. The interleukin-1 (IL-1) super family is one of the most important families of cytokines. Other members of the IL-1 super family are; IL-1α, IL-1β, IL-1 receptor antagonist (IL-1RA), IL-18 (1), and IL-33 (2). The IL-1 family members are shown in Table 1. High levels of IL-1β have been implicated in uncontrolled inflammation and fever in inflammatory diseases, including; familial Mediterranean fever (FMF), cryopyrin associated periodic syndrome (CAPS), Behcet's disease, and systemic onset juvenile idiopathic arthritis (SoJIA) (3). The underlying specific genetic causes for these diseases have not yet been elucidated due to inferring factors such as; high levels of IL-1β in the blood, and the etiology as well as manifestations of the diseases. This review discusses the implications of IL-1β and IL-18 production pathways in the development of SoJIA and FMF diseases.
| Family Name | Old Name | Property | Chromosomal Location |
|---|---|---|---|
| IL-1F1 | IL-1α | proinflammatory | 2p14 |
| IL-1F2 | IL-1β | proinflammatory | 2q14 |
| IL-1F3 | IL-1Ra | antagonist for IL-1α, IL-1β | 2q14.2 |
| IL-1F4 | IL-18 | proinflammatory | 11q22.2-q22.3 |
| IL-1F5 | IL-36Ra | antagonist for il-36α, il-36β, il-36γ | 2q14 |
| IL-1F6 | IL-36α | proinflammatory | 2q12-q14.1 |
| IL-1F7 | IL-37 | anti-inflammatory | 2q12-q14.1 |
| IL-1F8 | IL-36β | proinflammatory | 2q14 |
| IL-1F9 | IL-36γ | proinflammatory | 2q12-q21 |
| IL-1F10 | IL-38 | unknown | 2q13 |
| IL-1F11 | IL-33 | th2 responses, proinflammatory | 9p24.1 |
2. Evidence Acquisition
2.1. Process of IL-1β Production
The innate immunity system has two kinds of receptors for the recognition and response to extracellular and intracellular hazardous molecules. Toll-like receptor molecules (TLR) sense extracellular microbial elements, such as peptidoglycans in the cell-wall. Recognition of the ligands by TLRs leads to the expression of a number of immune and inflammatory proteins. The second receptor type is Nod-like receptors (NLRs), NLRs respond to various endogenous cellular molecules. They also recognize pathogens that pass through the cell barrier and enter into the cytoplasmic space. NLRs are cytoplasmic analogs of TLRs. Endogenous substrates for NLRs are comprised of toxins, defective nucleic acids, and normal cell debris. NLRs exhibit three domains including an N-terminal effecter domain (4), an intermediary nucleotide-binding domain; and a C-terminal leucine rich repeat (LRR) domain. LRR domains are short repeat motifs consisting of 22–28 residues of leucine that are found in a variety of cytoplasmic, membrane, and extracellular proteins, such as ribonuclease inhibitors and Toll-like receptors. NLR family genes are classified based on their N-terminal effector domain. Nod-like receptors (NALPs) are members of the NLR subfamily that have a pyrin domain (PYD) in the N-terminal of the molecules. Interactions between TLRs and NALPs lead to the production of pro-IL-1β and other inflammatory cytokines, including IL-18 and IL-33 (5-7). NALP3 has received the most attention, and it has been demonstrated that NALP3 is involved in the recognition of numerous exogenous and host molecules (8). The NALP3 inflammasome mediates activation of inflammatory caspase-1. The different components of the NALP3 inflammasome are; NALP3 itself, CARDINAL, ASC, and caspase-1 (9). In turn, caspase-1 cleaves pro-IL-1β to IL-1β, thus leading to the activation of IL-18 and IL-33. This vicious cycle leads to the maturation of some of the interleukin-1 family members, in particular IL-1β (10, 11). Moreover, TLR ligands, such as lipopolysaccharides, induce activation of nuclear factor κB, thereby triggering gene expression and synthesis of pro- IL-1β (8).
2.2. IL-1β Regulation and Function
IL-1β, which is a pyrogenic cytokine, is produced by monocytes, macrophages, fibroblasts, and dendritic cells. IL-1β is normally produced in infection, injury, or problematic immunologic situations (9). At basal concentrations, it causes; fever, hypotension, and the production of other proinflammatory cytokines, such as IL-6. IL-1β also activates IL-1 receptors on endothelial cells and that may cause the production of IL-6 and a cutaneous rash (12). In addition, IL-6 stimulates hepatocytes and induces the production of several acute-phase proteins, including C-reactive proteins and serum amyloid A (13). IL-1 receptors in the brain also activate the thermoregulatory center of the hypothalamus and lead to fever.
The synthesis and release of IL-1β is tightly regulated. The immune system uses several mechanisms to regulate excessive production of IL-1β, from transcription to post-translational modification (14). Several proteins are believed to regulate IL-1β production by interfering with the recruitment of caspase-1 or by directly disrupting caspase-1 activity. Some of these proteins are CARD-only proteins (COP), Iceberg, the caspase-1 inhibitor proteinase inhibitor 9 (PI-9), and the most important one here, pyrin (15-17).
2.3. IL-18 and Inflammation
IL-18 is one component of the IL-1 superfamily, thus it shares the same receptor with IL-1. It is produced by macrophages, dendritic cells and epithelial cells all over the body. The IL-18 receptor has two chains, including IL-18R_ α and IL-18R_ β. The IL-18 receptor binding site to the α chain of the receptor is similar to the IL-1 receptor type I (18, 19). The intracellular chain of the IL-18 receptor contains a Toll domain which phylogenetically link IL-18 to the Toll-like receptors. As mentioned before, TLRs are inducers of the NF-kB pathway which is responsible for pro IL-1 production. A natural IL-18 binding protein (IL-18BP) is a molecule that effectively neutralizes IL-18 activity. IL-18BP is a constitutively secreted, high-affinity and specific inhibitor of IL-18, in addition, alternate mRNA splicing of IL-18BP results in four isoforms (20, 21). The ‘A’ isoform is present in the serum of healthy humans at an excess 20-fold molar compared with IL-18 (22). Highly elevated levels of IL-18 have been reported in inflammatory disease (23) for instance serum levels of IL-18 were significantly higher in systemic JIA patients (24).
2.4. Pyrin as a Mediator of IL-1β Regulation
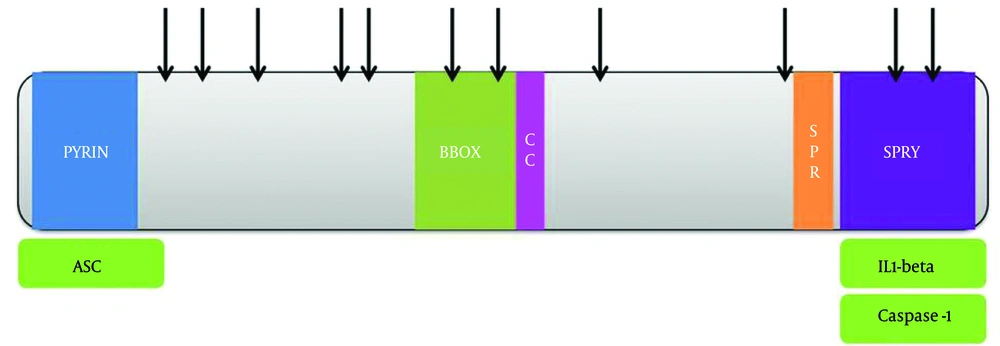
MEFV is the gene coding pyrin. Mutations in this gene cause familial Mediterranean fever, a recessively inherited systemic autoinflammatory disorder characterized by short recurrent bouts of fever and serosal, synovial, or cutaneous inflammation, and sometimes this leads to AA amyloidosis (25). Pyrin (or marenostrin), is a 781-aa protein expressed in neutrophils, eosinophils, monocytes, dendritic cells, and synovial fibroblasts (26). Pyrin contains a pyrin domain (PYD) at the N-terminus, which is a member of the death effector-fold domains (10, 27, 28), two B-boxes and a coiled-coil domain (BBCC) in the middle, as well as a SPRY domain in the C-terminus (also called a B30.2 domain). Pyrin interacts with ASC (29) through homotypic interaction of their N-terminal PYD domains (29). The C-terminal SPRY domain of pyrin binds to the catalytic domains of caspase-1 and inhibits enzyme activity (30, 31). Both the BBCC and the SPRY domain of pyrin interact with proIL-1β, although the interaction with the SPRY domain was clearly stronger. Based on researcher findings, pyrin interaction with pro IL-1β depends on the prodomain of pro IL-1β, because after maturation of IL-1β, this interaction cannot be detected. The inhibitory effects of pyrin on the IL-1β production pathway have been reported in caspase-1 self-activation, and an inhibitory effect of the SPRY on ASC dependent activation of caspase-1 and IL-1β processing (Figure 1). Increased processing and secretion of IL-1β was observed in cells in which pyrin was absent. It is proposed that the FMF mutations may affect binding of an unidentified inhibitory interaction partner, or affect the stability or localization of pyrin, and in this way it leads to a higher level of secreted IL-1β and intensified inflammatory responses, as observed in FMF patients. Pyrin appears to play an effective role in the regulation of the systemic inflammatory response, and the correlation of high levels of MEFV expression with adverse outcomes in critically ill children with multiple organ dysfunction syndrome confirms this association (32).
2.5. Systemic Juvenile Idiopathic Arthritis and IL-1
In 1897, George Frederic Still summarized his observations on 12 children with systemic onset of juvenile idiopathic arthritis (SoJIA) (33). Systemic juvenile idiopathic arthritis is one of the subtypes of juvenile idiopathic arthritis (JIA). While HLA associations are significant genetic factors in most arthritis subtypes and our previous research prove this relationship, there are few or no associations with SoJIA (34-37). Cases of SoJIA in adolescents are rare and adult onset is reported in only a few cases. In Europe, the incidence of JIA is about 10:100 000/y of which SoJIA represents 6-20% (38, 39). The International League of Associations for Rheumatology classified SoJIA as an arthritis in children starting before 16 years-of-age with symptoms such as; a daily quotidian fever of 39˚C (or more), persisting for more than two weeks, and at least one of the following clinical symptoms: an evanescent rash, lymphadenopathy, hepatosplenomegaly or serositis (40, 41). The outstanding clinical feature that distinguishes SoJIA from other subtypes of JIA is a fever up to more than 39˚C. The second important clinical sign in SoJIA is a cutaneous rash. The serum of SoJIA patients induces the transcription of genes in the innate immune system, including IL-1, in peripheral blood mononuclear cells (PBMCs). In addition, activated monocytes from patients with SoJIA secrete significantly higher amounts of IL-1β in comparison with the monocytes of healthy controls (42). IL-18 also shows very high serum concentrations, with high specificity for SoJIA, compared with other forms of JIA (43, 44). IL-1 acts on the bone marrow and stimulates granulopoiesis resulting in neutrophilia of the peripheral blood. IL-1 receptors in the brain activate the thermoregulation of the hypothalamus and lead to fever. IL-1β also activates IL-1 receptors in endothelial cells that may cause a cutaneous rash in SoJIA and result in the production of IL-6 (45). IL-6 on the other hand stimulates hepatocytes and induces the production of several acute-phase proteins, like C-reactive protein and serum amyloid A. Serum levels of IL-6 are markedly elevated in patients with SoJIA and this is correlated with systemic features of the disease (13), therefore, IL-6 concentrations in serum are a reflection of IL-1 activity in vivo.
The uncontrolled activation of inflammasomes, and cleavage of pro-IL-1 by caspase-1, have been shown to be important molecular mechanisms in different inherited autoinflammatory syndromes, resulting in spontaneous fever attacks (46, 47). Thus, the pathogenesis of SoJIA shows more similarities with auto-inflammatory diseases than with classical antigen-driven auto-immune diseases. No mutations that affect IL-1β regulation have so far been identified in SoJIA patients. However, speculation remains that susceptibility to SoJIA might also involve mutations in particular inflammasome components. Dysregulation of IL-1β production, IL-1Ra proteins, IL-1 type II decoy receptor proteins (48), or the soluble IL-1R accessory protein (IRAP) (49), might contribute to increased activity of IL-1β in SoJIA. The responsibility of IL-1β, and other cytokines for the pathogenesis of SoJIA, can be useful for using cytokine measurement of serum as a diagnostic instrument which can be more effective and low cost than measurements of CRP and ESR. Another benefit is in treatment. Anakinra, a recombinant IL-1 receptor antagonist (IL-1RA), is a new targeted drug which neutralizes the adverse effects of IL-1 superfamily members, such as IL-1β and IL-18, by blocking their receptors (50, 51).
3. Conclusions
Mutations in the MEFV gene, that are responsible for FMF disease, have been seen in SoJIA patients and our recent study shows mutations in this gene in Iranian patients (results not published). Pyrin is one of the IL-1 pathway regulators, and increased processing and secretion of IL-1β was observed in cells in which pyrin was absent. We speculate that it could be a major factor involved in the pathogenesis of SoJIA and suggest checking the effect of this mutation in MEFV gene sequence and expression of pyrin on IL-1β production in SoJIA patients. This proven relationship in the pathogenesis of SoJIA and FMF can be useful for making more effective and low cost diagnoses and it could also be a way to develop new molecular and efficient treatments for these diseases.