1. Introduction
H syndrome (OMIM 612391) is possibly an autosomal recessive disease with certain features in different organs. Mutations in SLC29A3 gene, encoding human equilibrative nucleoside transporter hENT3, lead to mononuclear cell skin infiltration and cutaneous manifestations (1). The described disorders in previously reported patients included hearing deficit, hyperpigmented indurated skin, hypertrichosis, heart anomalies, short stature due to growth hormone deficiency, hepatosplenomegaly, hypergonadotrophic hypogonadism, gynecomastia, hallux valgus, camptodactyly, proptosis with normal thyroid function tests, scrotal mass, hypertriglyceridemia, hyperglycemia and dilated scleral vessel angiopathies such as varicose veins and facial telangiectases. Less frequent findings include lateral tibial torsion, myelofibrosis, more severe skin involvement and absence of skin involvement in some other cases (1, 2).
Molho-Pessach et al. identified this disorder and reported H syndrome in 9 male and 1 female patients, who belonged to 6 consanguineous Arab families residing in villages near Jerusalem (2).
The same disease has been found in six Bulgarian patients and also in other parts of the world (3, 4). Gene prevalence was 1% in population of the mentioned Arab patients. This gene was not detected in European, Bulgarian, and Jewish control populations (1). SLC29A3 mutation in Arab patients was not the same as that in Bulgarian patients (1). It is important to know about this syndrome because of diversity of its clinical manifestations, especially in patients with an Arab origin.
2. Case Presentation
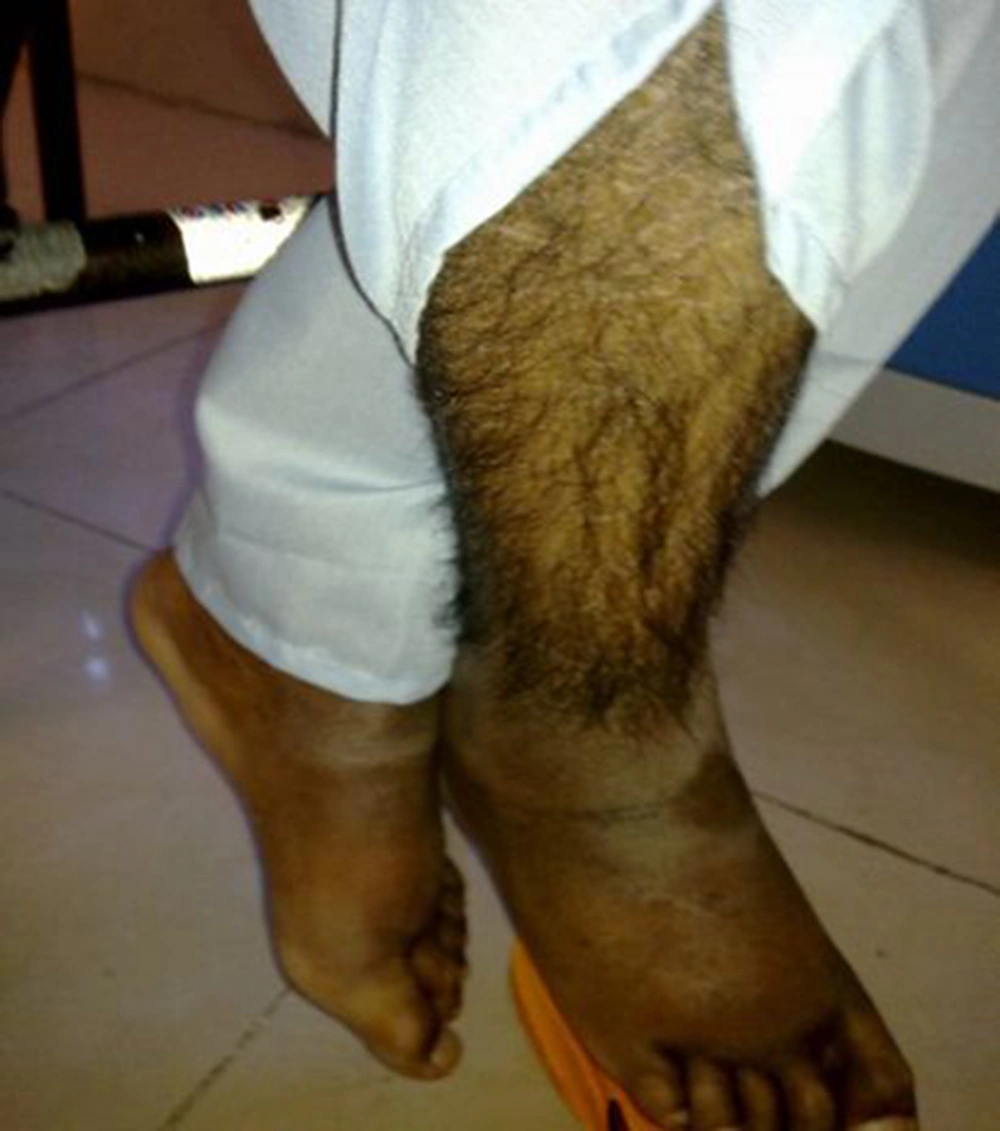
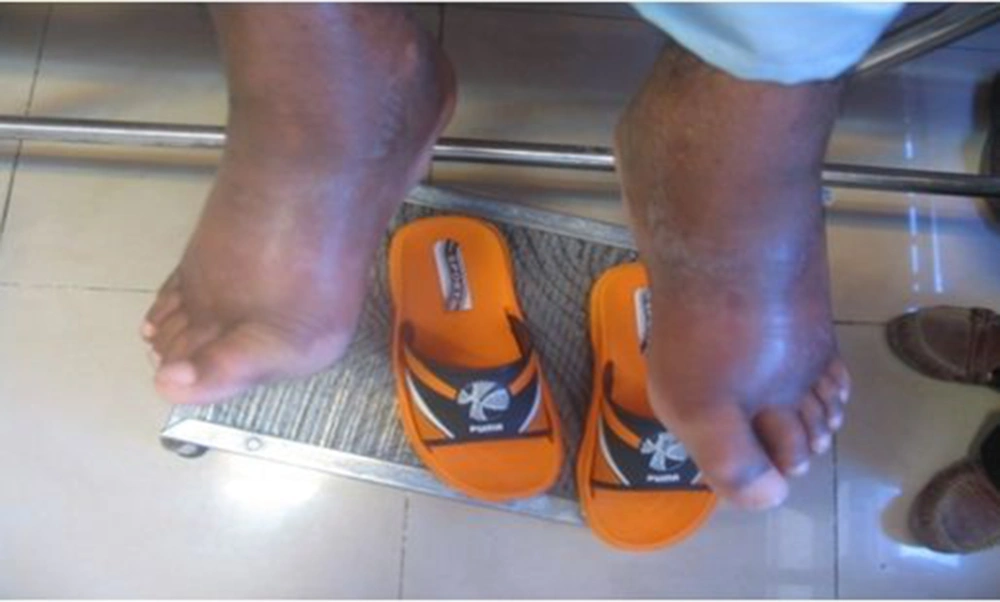
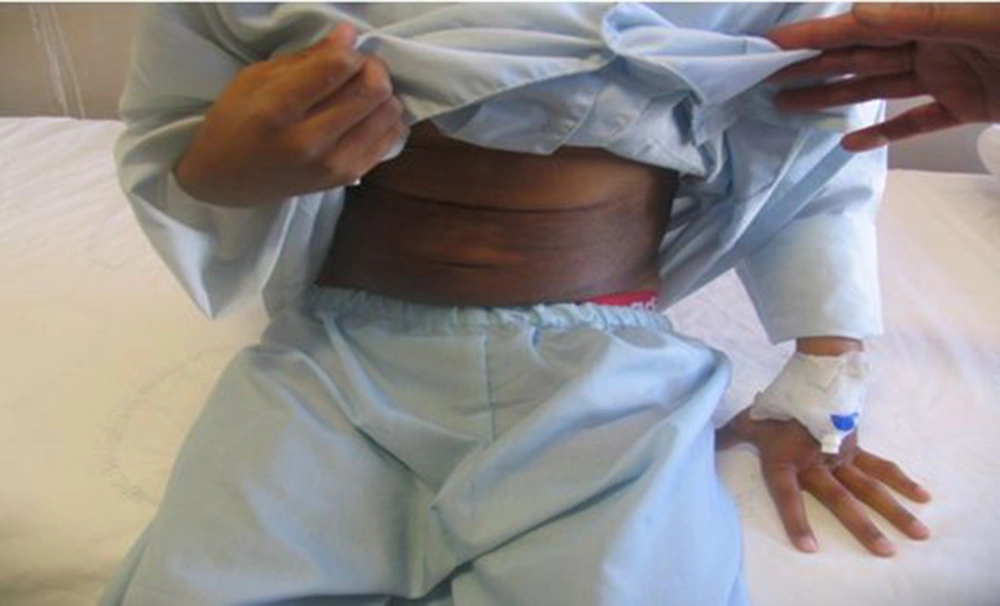
A 17-year-old boy was referred to our center, Namazi hospital, an academic center affiliated to Shiraz university of medical sciences, Shiraz, Iran. He had hyperpigmented and hypertricotic lesions and indurated seborrheic keratosis-like cutaneous patches mainly involving the extremities that have progressed slowly to abdomen and trunk (Figures 1, 2 and 3) and the patient's chief complaint was malaise and weakness. The onset of cutaneous lesions had begun since one year of age. No remarkable abnormal medical history was concerned. Regarding family history, his sister had an isolated hearing loss without any other abnormal manifestations. The patient’s parents belonged to a consanguineous family with Arab origin, from suburbs of Shiraz. Additional systemic features included sensorineural hearing loss, short stature, growth retardation, moderate mental retardation, delayed puberty, microphallus, moderate exophthalmos with normal thyroid function test, blue ring in iris, dirty sclera, hallux valgus with mild flexion contracture of toe joints, disability to walk, non-pitting edema in extremities, mild hepatomegaly and bilateral inguinal masses. Box 1 shows the most common observed manifestations of H syndrome, according to Molho-Pessach et al. study (5).
| Clinical Manifestations |
|---|
| Hyperpigmented skin patches (occasionally with hypertrichosis) |
| Sensorineural hearing loss |
| Short stature |
| Hepatomegaly/splenomegaly |
| Cardiac anomalies |
| Inguinal/genital masses |
| Hypogonadism with microphallus |
| Delayed puberty |
| Moderate exophthalmos |
| Mental retardation |
Echocardiography revealed dextrocardia, situs inversus and moderate pulmonary arterial hypertension. Abdominal ultrasound demonstrated mild hepatomegaly and bilateral oval inguinal masses with central echogenic fat. Right and left testes were normally palpated in scrotum. Before establishing the diagnosis and because of the complexity of the clinical manifestation, we screened the brain to find any coexistent anomaly. Brain magnetic resonance imaging (MRI) had normal findings. Bone marrow aspiration was not performed for patient.
Laboratory tests of our patient revealed a normochromic normocytic anemia with a high reticulocyte production index (RPI = 20) and thrombocytosis, but normal white blood cell (WBC = 10400/mm3 PMN= 68%, LYM = 26%) count. In complete blood count (CBC) hemoglobin (Hb) was 5.2 g/dL, MCV (mean corpuscular volume) 88fl and PLT (platelet count) 900000/mm3.
Direct Coombs test was 2+ positive and erythrocyte sedimentation rate (ESR) was 78. Our case did not show any glucose or lipid profile abnormalities. Liver function tests and bilirubin level were within the normal ranges. Patient’s lactate dehydrogenase (LDH) and glucose-6-phosphate dehydrogenase (G6PD) levels were measured to rule out hemolysis. The serum gonadotropin levels were not measured.
3. Discussion
Literature review shows cases from different geographical and ethnic backgrounds with clinical manifestations consistent with H syndrome (2, 6). Genetic evaluations found a mutation in SLC29A3 gene. The normal product of the wild type allele of this gene functions in nucleoside transport. Nucleoside transport is a vital function in cells that are unable to produce their nucleosides de-novo. Monocyte-like cells are one of these cells (7-9). The mutation causes a metabolic problem in such cells and dermal infiltration by them in immunohistochemical staining of skin (7).
Many in vitro and in vivo studies demonstrated a function of the gene in mitochondrial, lysosomal and cell membrane activities (10, 11). Because cell membrane, lysosome and mitochondria are ubiquitous in human body, phenotypic manifestations can be systematic and diverse (12, 13). Patients with mitochondrial defects have cardiomyopathy, sensorineural hearing loss, hepatomegaly, anemia, low height, hyperpigmentation, hypertrichosis and other common features of H syndrome (14, 15).
Mutations in SLC29A3 gene have been detected in patients with a mild phenotype. This finding suggests that many mild cases of H syndrome are not diagnosed and this syndrome is a rather common disease (1).
A case series reported 10 cases of H syndrome from 6 Arab consanguineous families with main features of hyperpigmented, hypertrichotic and indurated cutaneous patches involving middle and lower parts of their bodies, short stature, sensorineural hearing loss, cardiac anomalies, hepatosplenomegaly and scrotal masses (2). Our patient had very similar characteristics including hyperpigmented, hypertrichotic and indurated cutaneous patches involving middle and lower parts of their bodies, short stature, sensorineural hearing loss, cardiac anomalies, hepatomegaly and scrotalmass.
Another case series also described cardiac anomalies such as ASD Secundum, VSD, mildly elevated pulmonary hypertension, Mitral valve prolapse and cardiomegaly (2). Our patient had some findings not previously described such as situs inversus and thrombocytosis. Elevated ESR was previously reported in other patients like ours. Patient’s lactate dehydrogenase (LDH) was measured to rule out hemolysis before establishing the diagnosis of the syndrome. The patient had immune hemolytic anemia (low hemoglobin, high RPI and positive coombs test) with a good response to prednisolone. Anemia was due to myelofibrosis and hemolysis. Microphallus was due to hypogonadism.
The typical cutaneous hyperpigmentation together with sclerodermatous thickening and hypertrichosis are the most common clinical features of H syndrome. These clinical signs are pathognomonic features for H syndrome (5). Laboratory evaluation in our patient was incomplete because of unwillingness of his parents to continue genetic and other laboratory evaluations and skin biopsy. Therefore, genetic confirmation of H syndrome was not possible and the diagnosis was made clinically by a strong association with aforementioned cases.
H syndrome should be considered in patients with a constellation of symptoms such as hyperpigmentation, hypertrichosis, cardiac anomalies, hepatosplenomegaly, hearing deficit, hypogonadism, short stature, flexion contracture of fingers and toes, microphallus and hypertriglyceridemia.