1. Background
Bacterial diarrhea is the second most common cause of morbidity and mortality in developing and underdeveloped countries and among the causative agents, Escherichia coli (E. coli) plays a dominant role in community acquired enteritis (1, 2). The E. coli is a type of bacteria that lives in the human intestine, as normal flora; however, five distinct pathotypes have been characterized, including enteroaggregative E. coli (EAEC), enteroinvasive E. coli (EIEC), enterohemorrhagic E. coli, enteropathogenic E. coli (EPEC), and enterotoxigenic E. coli (ETEC) that cause diseases (3). The EAEC is an important etiologic agent of diarrhea among children and is increasingly recognized as a global emerging pathogen, with potential threat to public health (4-6). It was first described in 1985, recognized by its distinctive adherence to HEp-2 cells in an aggregative, stacked brick-like pattern, and also its tendency to attach to abiotic surfaces, when grown in tissue culture plates (7, 8). This bacterium produces a watery diarrhea and can cause symptoms, such as abdominal pain and fever, in children, as well as adults. Most prominently, besides causing acute diarrhea, it can also persist in the human intestine subclinically, inducing chronic inflammation in the absence of dysentery (9). In addition, the pathogenic potential of EAEC has been demonstrated by the emergence of recent food borne outbreaks, most notably in Germany in 2011 (10). The frequency of diarrhea in developed and developing countries differs and poor sanitation and crowded living conditions increase the propensity for EAEC to spread in developing countries. The EAEC is also linked to diarrhea in adults, including HIV‐positive patients and travelers visiting less developed regions of the world. Recently, it was found that EAEC isolates are associated with extraintestinal infections, such urinary tract infections (UTIs) and hemolytic uremic syndrome (HUS) (11, 12).
At the molecular level, a diagnostic probe was initially constructed for polymerase chain reaction (PCR) amplification of a 630-bp fragment of a plasmid encoded autotransporter adherence pCVD432 (aatA) gene, in harboring strains (13). Principally, among adherence genes, fimbriae I and II (AAF/I and AAF/II), which are required for attachment to surfaces and form biofilm, as well as the AAF/III that help EAEC strains bind loosely to epithelial cells, were identified. The AAF was assigned into several distinct alleles, including four major pilin variants (aggA, aafA, agg3A and agg4A) (14).
Similarly, plasmid encoded serine protease autotransporters of Enterobacteriaceae (SPATE) toxins detected in several EAEC strains that cause increased mucus release, exfoliation of cells and development of crypt abscesses (15). This family comprises two classes; class-I consists of the cytotoxic genes, which include pet and sat (16) and class-II include the genes involved in colonization (pic) and Shigella extracellular protease (sepA), which appears to be involved in tissue invasion (17). In addition, several strains of EAEC produce enterotoxins, such as the enteroaggregative heat stable toxin-1 (EAST1) and the Shigella enterotoxin-1 (ShET1) (18).
Little information is available on the prevalence of EAEC infections in Iran. A PCR method, based on identifying the presence of the virulence genes pCVD432, aggR, aggA, aafA, aap, and astA was used among EAEC isolated from fecal samples, in Tehran (19). In another study from Iran, out of 715 stool samples collected from patients showing diarrhea in Shiraz, 101 diarrhoeagenic E. coli were identified, five of which confirmed as EAEC (20). Similarly, Bouzari et al. (21) isolated 98 E. coli strains that displayed the aggregative adherence pattern on HeLa cells and hybridized with the pCVD432 probe.
2. Objectives
The aims of present study were to investigate distribution of the putative plasmid encoded AAF (AAF/I, II, III), SPATE cytotoxins (sat, sepA, pic), Shigella like enterotoxin-1 astA genes and biofilm formation among EAEC strains, isolated from stool of children with diarrhea, in south east Iran.
3. Patients and Methods
3.1. Specimen Collection and Microbiological Processing
A total of 464 stool samples of diarrheal patients referred to three main hospitals were collected by an expert laboratory technician during the summer seasons of 2013 and 2014. The children were classified according the age groups: 1-5 years old, 5-10 years old and more than 10 years old. Initially screened for ‘presumptive’ colonies of E. coli by overnight enrichment of the samples in the E. coli enrichment broth (HiMedia, Mumbai, India), followed by streaking on selective MacConkey and eosin-methylene blue (EMB) agars (HiMedia, Mumbai, India) and incubation of plates at 37°C for 24 hours and final bacterial identification, colonies were recovered and kept at freezing temperature (-70°C) for further analyses.
3.2. Detection of Aggr and Cdv432 Genes by Duplex- Polymerase Chain Reaction
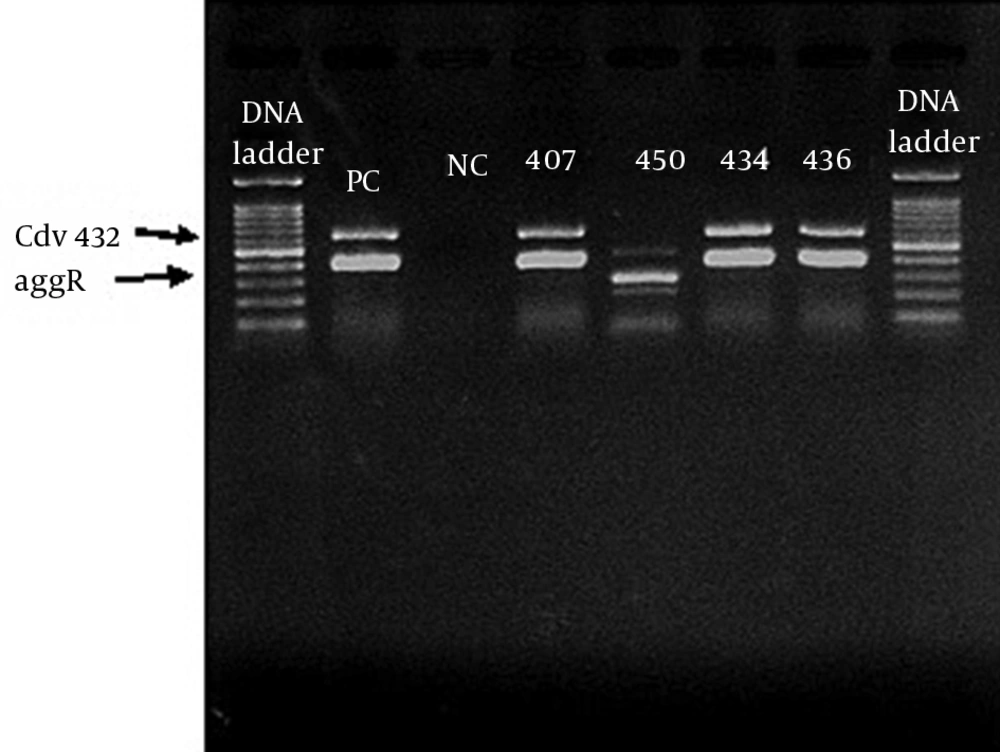
The E. coli isolates were tested for the presence of aggR and pCVD432 genes, using duplex-PCR, as described previously (13). Briefly, total genomic DNA was isolated from a single colony grown on EMB agar plates by boiling method and used for PCR reactions. The forward and reverse primer sequences (Thermo Scientific, Vilnius, Lithuania) for EAEC-specific genes were designed using references, as shown in Table 1 and confirmed by online Primer Quest software tool (http://www.ncbi.nlm.nih.gov/GeneBank). To verify whether the PCR sensitivity is sufficient for diagnostic use, ten-fold dilutions of the template DNA of diarrheagenic E. coli was quantified, using optical density at 280/260 nm. The PCR reaction was performed with 2 µL DNA template, primers (20 pM) 1 µL, master mix 12.5 µL (Amplicon, Brighton, UK), DD/W 9.5 µL in a temperature gradient thermal cycler (Biometra-T gradient, Biometra GmbH, Gottingen, Germany). Amplification protocol comprised of initial denaturation temperature at 95°C for 5 minutes, followed by 30 cycle of denaturation at 95°C for 40 seconds, annealing at 56°C for 40 seconds, extension at 72°C for 30 seconds, followed by a final extension at 70°C for 5 minutes. The electrophoresis of the amplified DNA fragments, along with 100 bp DNA marker ladder was carried out using 1.2% agarose gel (Merck, Darmstadt, Germany) in 1.6 × tris-borate-EDTA (TBE) buffer (pH 8.2) and bands were visualized by UV gel documentation system. A reference DNA from prototype strain EAEC 042 containing both the aggR and pCVD432 genes was run as positive control strain, while E. coli K12 DH5α DNA was used.
3.3. Detection of Enteroaggregative Escherichia Coli Virulence Genes by Multiplex-Polymerase Chain Reaction
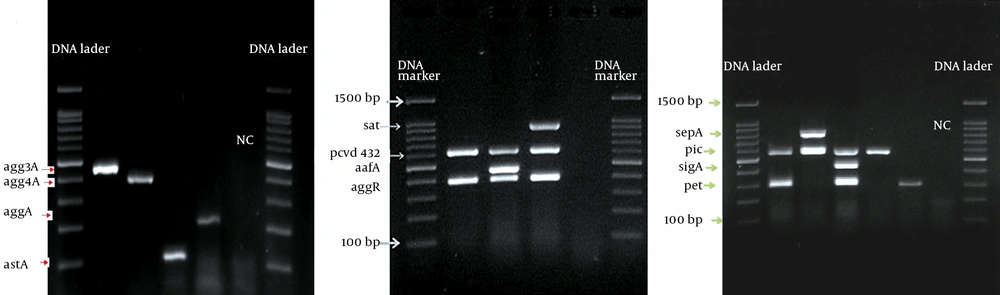
All EAEC positive samples were analyzed by additional multiplex PCR (M-PCR), with the primers and conditions described in Table 1 and Table 2, to amplify fragments of 10 genes encoding putative virulence factors (agg4A, aggA, aafA, agg3A, astA, satA, sigA, pet, pic and sepA). The M-PCR was divided into three sets of multiplex reactions, each with a specific denaturation and annealing temperature. A control for each virulence gene was also run along the tests.
3.4. Hemolytic Activity on Sheep Blood Agar
An amount of 200 µL of the EAEC culture was streaked on blood agar base medium (Merck, Darmstadt, Germany) supplemented with fresh sheep blood (5%). The plates were then incubated for 24 hours at 37°C and hemolytic activity surrounding the colonies was determined.
3.5. Biofilm Formation
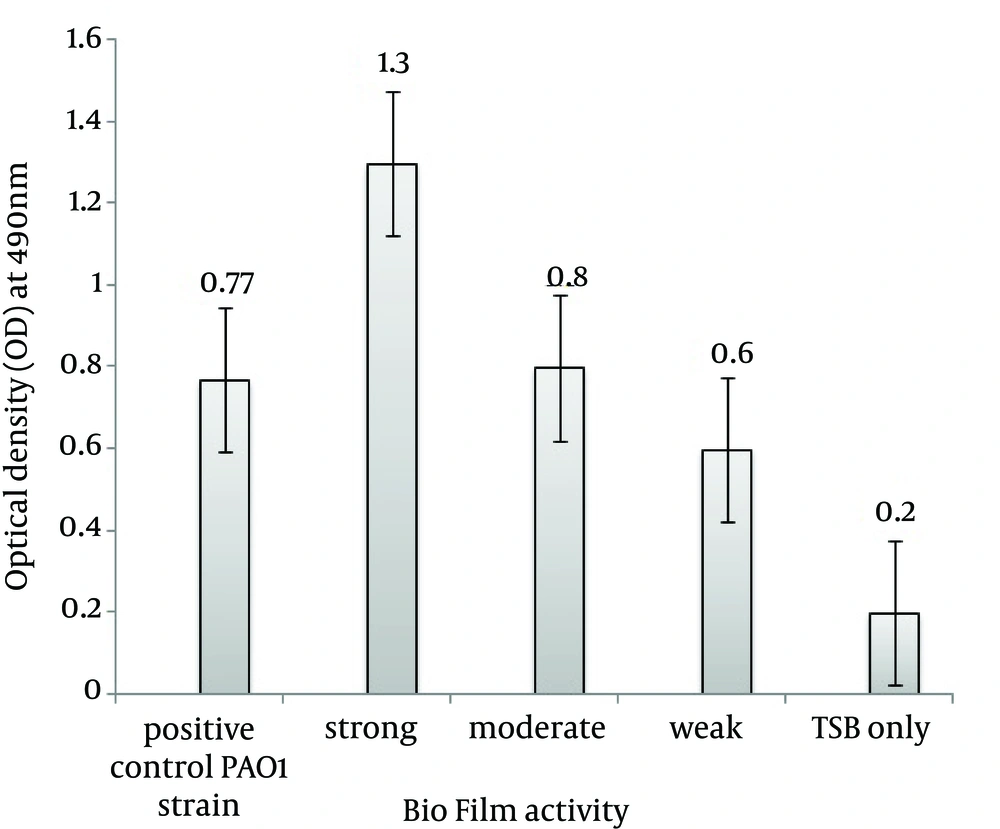
Formation of the biofilm for each EAEC isolate was quantified by microtiter method (22), with a number of modifications. Briefly, one loopful from each well of isolated colony was inoculated into a sterile TSB medium (2 mL), containing glucose (1% W/V), to optimize biofilm formation [glucose was sterilized separately by filtering, using 0.25 µM membrane filter (Sartorius AG, Goettingen, Germany)]. The optical density (OD) of 650 nm was then adjusted to 0.13 to reach 0.5 McFarland standard (1.5 × 108 CFU/mL), followed by further dilution of prepared bacterial suspension to reach ~ 106 CFU/mL. A quantity of 100 µL of the standardized cell suspension was transferred to the wells of a round-bottom 96-well microtiter plate. Bacteria were allowed to adhere and grow without agitation for 24 hours at 37°C. After incubation, non-adherent cell suspensions were aseptically aspirated with sterile microtips, washed and replaced with 20 µL of sterile phosphate buffered solution (pH 7.2) to remove any remaining suspended cells. In order to fix the biofilm, 150 µL of methanol were added to each well and kept at room temperature (25°C) for 20 minutes. Methanol was removed and replaced with 200 µL methylene blue (1% W/V) and incubated for 30 minutes at room temperature. Microplate wells were rinsed twice with PBS pH 7 and 200 µL glacial acetic acid (33% V/V) were added. The optical density was measured at 490 nm, by using Synergy 2 multi-mode microplate reader (BioTek, Winooski, VT, USA). For each experiment, correction for background staining was made by subtracting the value for methylene blue bound to uninoculated controls. Positive standard for biofilm formation was Pseudomonas aeruginosa PAO1, and negative control was TSB broth only. The mean value was calculated from three independent experiments. The isolates were then classified into strongly adherent (strong biofilm), moderately adherent (moderate biofilm), weakly adherent (weak biofilm) and not adherent (no biofilm), based on the formula described by Stepanovic et al. (23).
4. Results
We classified children with diarrhea into three anthropometric groups. Group one had highest proportion in the population [56.52% (n = 13)] and had an age range of 1-5 years old. The second group [21.73% (n = 5)] consisted of children with ages between 5-10 years old, while the third group [21.73% (n = 5)] included patients ≥ 10 years old.
A total of 23 strains of EAEC were detected by duplex-PCR, which harbored both the aggR and pCVD432 genes (Figure 1). Distribution of virulence genes and toxins in EAEC isolates is presented in Table 2. Four major EAEC fimbrial variants, aggA, agg4A, agg3A and aafA, were detected with the frequency of 21.7% (n = 5), 26.8% (n = 6), 21.7% (n = 5) and 4.3% (n = 1), respectively (Figure 2 A, 2B). In addition, we identified the class I and II SPATE genes including pic, sat, sepA, and pet, in 56.5% (n = 13), 30.4% (n = 7), 26.8% (n = 6) and 21.7% (n = 5) of the EAEC population, respectively (Figure 2 C). SigA gene was found in only one isolate (4.3%), while the heat-stable enterotoxin-1 (EAST1) astA gene was detected in four (17.3%) cases (Figure 2 A and Table 2). Pic-sat-sepA-agg4A was the most prevalent gene combination detected in our study (Figure 2 D). A total of 56.4% of the isolates were α-hemolytic, while the remaining isolates were non-hemolytic. Quantitative evaluation of the biofilm of the represented strains introduced six isolates with strong (severely adherent), 11 isolates with moderate (moderately adherent), and six isolates with weak (weakly adherent) biofilm (Figure 3).
| Virulence Gene | Properties of Target | Primer Sequence (5´ - 3´) | Amplicon Size, bp | References |
|---|---|---|---|---|
| Sat | Secreted autotransporter toxin gene | F-TCA GAA GCT CAG CGA ATC ATT G; R-CCA TTA TCA CCA GTA AAA CGC ACC | 930 | Boisen et al. (2009) (18) |
| SigA | Class I cytotoxic SPATE protein | F-CCG ACT TCT CAC TTT CTC CCG; R-CCA TCC AGC TGC ATA GTG TTT G | 430 | Boisen et al. (2009) (18) |
| Pet | Plasmid encoded cytotoxin | F-GGC ACA GAA TAA AGG GGT GTT T; R-CCT CTT GTT TCC ACG ACA TAC | 302 | Restieri et al. (2007) (17) |
| Pic | Serine protease autotransporter toxin | F-ACT GGA TCT TAA GGC TCA GGA T; R-GAC TTA ATG TCA CTG TTC AGC G | 572 | Restieri et al. (2007) (17) |
| SepA | Shigella extracellular protein A | F-GCA GTG GAA ATA TGA TGC GGC; R-TTG TTC AGA TCG GAG AAG AAC G | 794 | Restieri et al. (2007) (17) |
| AafA | AAF/II fimbrial subunit | F-CAG AAT GTT TGC GAT TGC TAC; R-TTT GTC ACA AGC TCA GCA TT | 468 | Boisen et al. (2012) (18) |
| agg3A | AAF/III subunit | F-GTA TCA TTG CGA GTC TGG TAT TCA G; R-GGG CTG TTA TAG AGT AAC TTC CAG | 462 | Boisen et al. (2012) (18) |
| agg4A | AAF/IV subunit | F-TCC ATT ATG TCA GGC TGC AA; R-GGC GTT AAC GTC TGA TTT CC | 411 | Boisen et al. (2012) (18) |
| AggA | Aggregative fimbria AAF/I subunit | F-TCT ATC TRG GGG GGC TAA CGC T; R-ACC TGT TCC CCA TAA CCA GAC C | 220 | Boisen et al. (2012) (18) |
| AstA | Heat-stable enterotoxin (EAST1) | F-ATG CCA TCA ACA CAG TAT AT; R-GCG AGT GAC GGC TTT GTA GT | 110 | Vila et al. (2000) (15) |
| AggR | Transcription of AAFs | F- ACGCAGAGTTGCCTGATAAAG; R-ATACAGAATCGTCAGCATCAGC | 308 | Restieri et al. (2007) (17) |
| pcdv432 (aatA) | Transcription of AAFs | F- CTGGCGAAAGACTGTATCAT; R- AAATGTATAGAAATCCGCTGTT | 630 | Boisen et al. (2012) (18) |
a Abbreviations: AAF, fimbrial subunits; EAEC, enteroaggregative Escherichia coli; SPATE, serine protease autotransporter toxins.
| EAEC, Isolates | SAPTE | Fimbrial Genes | Enterotoxin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Class I | Class-II | (AAF) | ||||||||
| pet | sat | sigA | Pic | sepA | aggA | agg3A | agg4A | aggfA | astA | |
| 43 | + | + | + | |||||||
| 110 | + | |||||||||
| 116 | + | + | ||||||||
| 147 | + | + | + | |||||||
| 166 | + | + | + | |||||||
| 188 | + | + | + | + | ||||||
| 300 | + | |||||||||
| 311 | + | + | + | + | + | |||||
| 316 | ||||||||||
| 335 | + | + | ||||||||
| 379 | + | |||||||||
| 383 | + | |||||||||
| 403 | + | + | ||||||||
| 407 | + | + | ||||||||
| 417 | + | |||||||||
| 428 | + | + | + | |||||||
| 434 | + | + | + | |||||||
| 436 | + | + | + | |||||||
| 437 | + | + | + | + | ||||||
| 438 | + | + | + | |||||||
| 450 | + | |||||||||
| 455 | + | |||||||||
| 463 | + | + | + | |||||||
a Abbreviations: AAF, fimbrial subunits; EAEC, enteroaggregative Escherichia coli; SPATE, serine protease autotransporter toxins.
5. Discussion
The EAEC strains are heterogeneous, both with respect to symptoms developed by infected hosts and with respect to virulence genes (24). In the present study, which was carried out for the first time in south east Iran, we demonstrated the importance of specific virulence genes in the biofilm formation of EAEC isolates. The rate of EAEC infection, in our case, was similar with reports from other parts of Iran (23, 24). Indeed, the varying presence of the different virulence factors indicates heterogeneity of the EAEC isolates. Although other studies have shown that the prevalence of pic varies between 4.6% and 57% depending on the strain (25), however, in our case, it was 56.52%. Similarly, in our study, the aafA gene was detected in only one isolate, followed by aggA, agg3A and agg4A, and may suggest that regional differences have a role in the prevalence of EAEC fimbrial adhesions. It has been reported that EAEC isolates were more likely to produce biofilm than non-diarrheagenic E. coli isolates and the production of biofilm was associated with the virulence (26). The pathogenic mechanisms of EAEC infection are only partially understood and are most consistent with mucosal colonization, followed by secretion of enterotoxins and cytotoxins. It was suggested that AggR protein may initiate a positive feedback loop that results in its own amplification, a mechanism that may partly explain the marked increase in aggR expression levels. This likely contributes to biofilm formation.
In conclusion, the results of our study, which carried out in south east Iran for the first time, clearly showed that the rate of infection by diarrhoeagenic EAEC was highest in children less than 5 years. The isolates harbored wide variety of virulence genes that help organism to attach to the intestinal epithelial cells and damage them. In addition to aggR and pCVD432, the pic, sepA and agg4A genes were more frequently involved in strong biofilm. However, the roles of these genes in the biofilm formation yet remain to be determined.