1. Context
1.1. The Clinical Case
Fred is a three-year-old boy who was born prematurely. His mother, who is single and works full-time, reported to us that since starting kindergarten a year ago, Fred has experienced monthly acute respiratory tract infections (RTIs) warranting medical attention and treatment. He is about to begin his final year of kindergarten, and his mother, who is affected by atopic eczema and asthma, is concerned about possible new acute RTIs and future complications. How should Fred’s case be managed?
1.2. Respiratory Tract Infections: Epidemiology and Burden of Disease
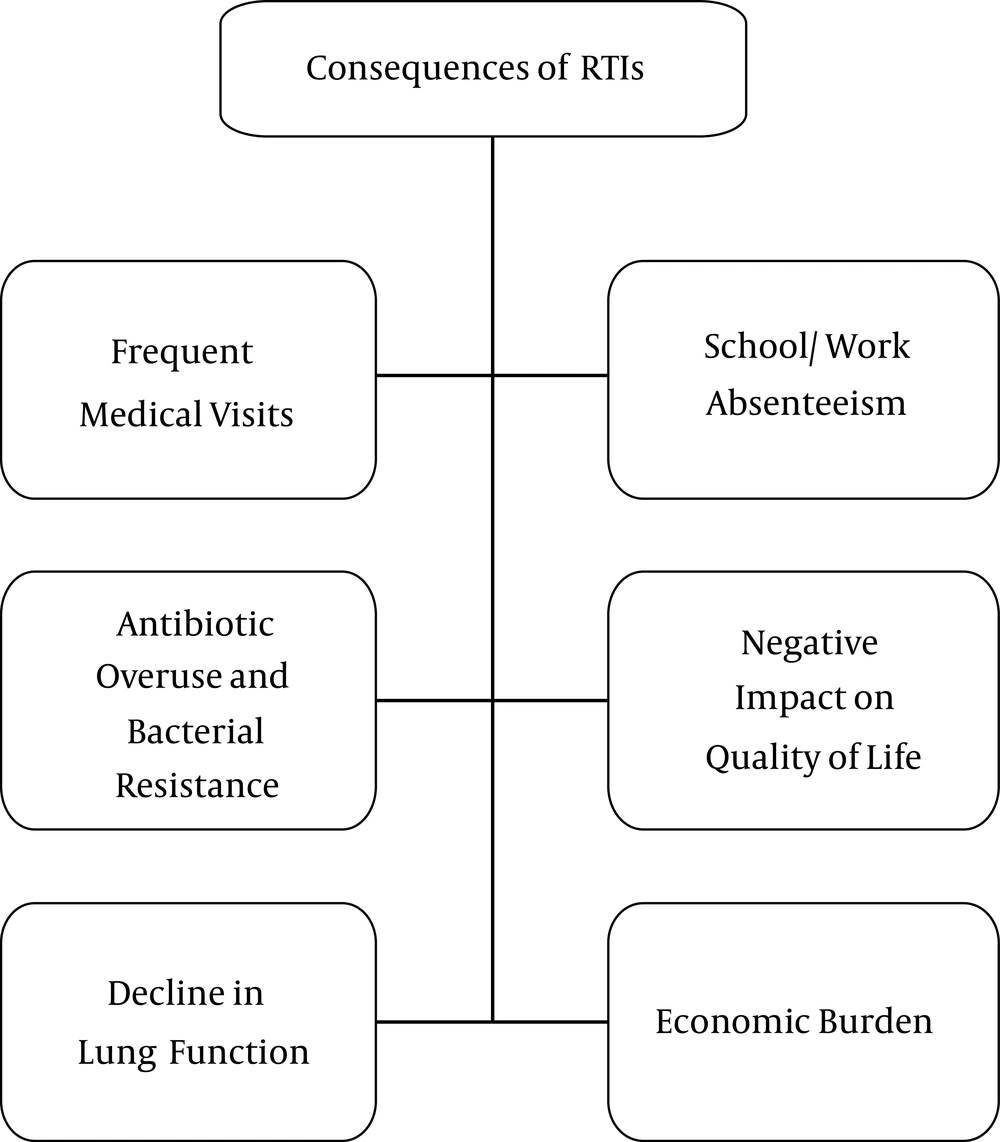
Worldwide, acute infections of the respiratory airways are associated with significant morbidity and mortality among pediatric patients (1). Recurrent RTIs are especially common in young children; in developed countries, they affect up to 25% of children under one year of age and 18% of children one to four years of age (2). In developing countries, RTIs are a leading cause of childhood mortality, resulting in over two million deaths per year (3, 4). Pediatric RTIs result in frequent complications that necessitate multiple medical visits (5-8). In developed countries such as Switzerland and Italy, nearly 50% of pediatric consultations are caused by RTIs (9-11) and in the United States, RTIs are among the leading causes of hospital admissions (12, 13). Despite the availability of healthcare in developed countries, RTIs nevertheless present a tremendous clinical and economic burden (14). Globally, recurrent pediatric RTIs pose a difficulty for patients’ families and a clinical challenge for treating physicians (Figure 1).
The main pathogens that trigger respiratory infections are viruses (such as the respiratory syncytial viruses, rhinoviruses, and influenza viruses) (15). Although viruses are often responsible for RTIs, bacterial super-infections commonly occur. Bacterial infections are observed in up to 60% of patients whose RTI symptoms last for 10 days or more (16). The most prevalent bacterial respiratory pathogens are species such as Streptococcus pneumoniae, Mycoplasma pneumoniae, Haemophilus influenzae and Streptococcus pyogenes (17). There is also evidence of a synergistic effect between viruses and bacteria in the pathogenesis of respiratory infections (18). A classic example is the synergism between influenza viruses and S. pneumonia (17). Although infection with influenza viruses alone may be fatal, mortality dramatically increases with a bacterial super-infection (17).
Several characteristics of RTIs contribute to the burden of illness. First, RTIs tend to recur in pediatric patients. The clinical sequelae of RTIs can result in long-term complications and further contribute to the disease burden, resulting in secondary infections, wheezing, and the development of asthma (6, 7). In the United States, pneumonia treatment costs an estimated $ 7 - 12 billion annually (12, 13). A recent Swiss study estimated that the overall cost associated with treatment of community-acquired pneumonia in children ages two months to 16 years was 11,258 CHF per episode, leading the authors to conclude that childhood pneumonia results in a significant medical cost burden that may be largely underestimated (19). Hospitalizations due to pediatric lower RTIs are especially pronounced in the winter months, thereby generating additional costs (20). This economic burden is projected to increase over the next 20 years, highlighting the importance of effective prevention strategies for reducing demands on the healthcare system (20). Pediatric RTIs require multiple medical visits and time spent at home for the patient and their caregivers, leading to school and/or work absenteeism and affecting the quality of life.
Another important consequence of pediatric RTIs stems from the fact that they are a leading cause of antibiotic prescriptions (21). Frequent antibiotic use, particularly for upper RTIs of viral origin, is common practice in ambulatory care (22, 23). Recent evidence shows that the number of broad-spectrum antibiotic prescriptions has increased even in cases for which no therapy is warranted or for which narrower-spectrum alternatives are appropriate (22, 23). It should be noted, however, that the appropriate tailoring of antibiotic agents may not always be possible in the primary care setting due to uncertainties in diagnosis and in identification of the infectious agent (24). Nevertheless, this misuse and overuse of antibiotics results in unnecessary drug-related adverse events, (25) contributes to antibiotic resistance and increased clinical failure, and incurs further medical costs (26, 27).
1.3. Recurrent RTIs: Definitions and Diagnosis
The most commonly used definition of respiratory tract infection is any upper or lower respiratory disease and any respiratory illness associated with fever (axillary temperature ≥ 37.5°C or rectal temperature ≥ 38°C) (28). Symptoms normally include at least one of the following: runny nose, nasal congestion, sore throat, cough, earache, wheezing, and/or shortness of breath lasting at least two to three days or more. Recurrent episodes should be separated by at least a two-week period with no symptoms.
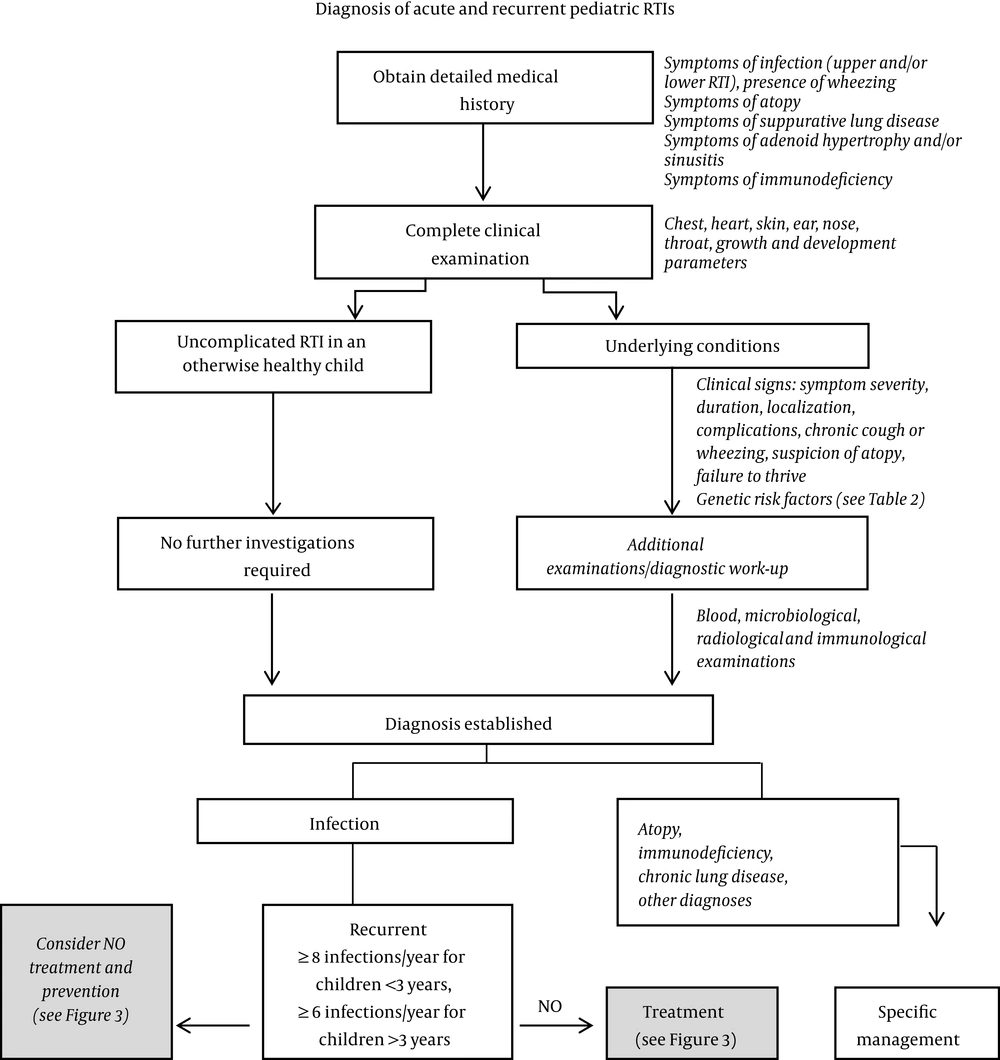
RTIs may be further classified into those that affect the upper or lower respiratory tracts. The majority of RTIs affect the upper respiratory tract, presenting as the common cold, tonsillitis, pharyngitis, laryngitis, rhinosinusitis, and otitis media. Although infections involving the upper respiratory tract are self-limiting and can be managed, infections of the lower respiratory tissues (tracheitis, bronchiolitis/bronchitis, and pneumonia) can have serious consequences that may lead to hospitalization and/or death (29). Thus, children like Fred who present with RTI symptoms are a diagnostic challenge. It is necessary to distinguish between patients for whom RTI symptoms have an uncomplicated cause (such as a viral infection) and those whose symptoms reflect a more serious underlying pathology (such as bronchiectasis or immune dysfunction) predisposing the patient to respiratory infections (30). Notably, there are several disorders (which may or may not be caused by a primary infection) whose symptoms resemble those of RTIs (Table 1) (31). The early symptoms of infection with measles and chickenpox are similar to those of RTIs (31). Another mimic is allergic rhinitis, characterized by excessive lacrimation and itchy eyes and manifested seasonally or following exposure to specific allergens. Sore throat may be indicative of acute thyroiditis, Ludwig’s angina, and gastroesophageal reflux disease, all of which should be differentiated from pharyngitis (31). Exclusion of pneumonia is a key diagnostic step when evaluating a patient with acute tracheobronchitis (31). If a cough lasts more than three weeks, then postnasal drip, asthma, and gastroesophageal reflux disease are the most likely causes (Table 1) (31). Therefore, the diagnosis of recurrent RTIs poses a clinical and diagnostic challenge with important implications for management strategies, as the role of the physician has evolved from disease treatment to health maintenance and disease prevention. A diagnostic algorithm for acute and recurrent pediatric RTIs is shown in Figure 2.
| Disease | Symptoms That Resemble RTIs |
|---|---|
| Measles, chickenpox | Similar symptoms to the common cold |
| Allergic rhinitis | Itchy eyes, excessive lacrimation |
| Acute thyroiditis, Ludwig’s angina, gastroesophageal reflux disease, drug-induced mucositis | Sore throat (pharyngitis) |
| Wegener’s granulomatosis | Sinusitis |
| Postnasal drip, asthma, gastroesophageal reflux disease | Cough |
Another difficulty is that there is no universal consensus on the definition of recurrent childhood RTIs. Furthermore, the number of episodes that are used to define recurrence varies according to the disease and severity. The most widely accepted definition is the occurrence of eight or more documented airway infections per year in preschool-aged children (up to three years of age), or of six or more in children older than three years of age, in the absence of any underlying pathological condition (32). Other definitions have been reported in the literature, such as three or more episodes per fall-winter period or over the course of six months (for two consecutive years) (33). Otitis media is considered recurrent if the patient experiences three episodes in six months or four episodes in 12 months, whereas infectious rhinitis is defined as recurrent if more than five episodes occur per year (34, 35). Pharyngitis or tonsillitis are considered recurrent if more than three episodes have been treated within a 12-month period (2, 34). Finally, infections of the lower respiratory tract are considered recurrent if more than three episodes occur within 12 months (36, 37). It is estimated that 10% - 15% of children experience recurrent RTIs (38). Our case study, Fred, likely falls into the category of preschool children with recurrent RTIs.
1.4. What are the Risk Factors for Recurrent RTIs?
Compared to healthy adults, infants and young children are at increased risk of recurrent RTIs due to the relative immaturity of their immune system. Humoral and cellular immunity do not mature until the fifth or sixth year of life (37, 39, 40) and the infant immune system is characterized by immature immune cell function and activation as well as an imbalance towards Th2 cytokines (41, 42). Immunoglobulin (IgG) subclass deficiency is often observed in young children, and 57% of children who have recurrent RTIs are deficient in one of the Ig subclasses, particularly IgG2 (29). Deficiencies of secretory IgA, alone or in combination with IgG subclass deficiency, also predispose an individual to recurrent RTIs (43). However, since the majority of these children do not have an underlying immunodeficiency (a rare condition), these findings reflect a transient immaturity of the developing immune system that results in greater susceptibility to RTIs (2, 29). Other unmodifiable risk factors are the presence of allergies, a family history of atopy, premature birth, and craniofacial abnormalities (Box 1) (30, 37, 44, 45).
| Physiological and genetic risk factors |
|---|
| Family history of atopy |
| Presence of allergies, atopy |
| Low birth weight or premature birth |
| Physiological abnormalities of the airways |
| Gastroesophageal reflux disease |
| Male gender |
| Craniofacial abnormalities |
| Environmental risk factors |
| Reduction or lack of breastfeeding |
| Attendance at daycare centers and early socialization |
| Large family size, school-aged siblings, overcrowding |
| Parental smoking, smoking during pregnancy |
| Malnutrition |
| Missing vaccinations |
| Physical stress, intense physical exertion |
| Climate and environmental factors (exposure to pollution) |
| Dampness in the home environment |
| Use of pacifiers |
| Bottle feeding in the prone position |
With his family history of atopy and the fact that he was born prematurely, Fred is at increased risk of RTIs compared to children who do not share these risk factors. RTIs occur more frequently during the winter months, partly due to overcrowding. Transmission of pathogens that cause RTIs occurs via aerosolized droplets or direct contact with contaminated secretions, with subsequent invasion of the eyes or respiratory epithelium (52). Thus, crowded conditions (such as schools and daycare centers) favor the transmission of respiratory pathogens (53, 54). Fred’s attendance at Kindergarten is an additional factor that puts him at risk of RTIs. Exposure to environmental factors such as air pollution and passive smoking are additional risk factors that can predispose a child toward RTIs (Box 1) (55). Other modifiable risk factors include pacifier use and bottle feeding in the prone position (Box 1) (46-48).
1.5. Recurrent RTIs: Rationale for Prevention
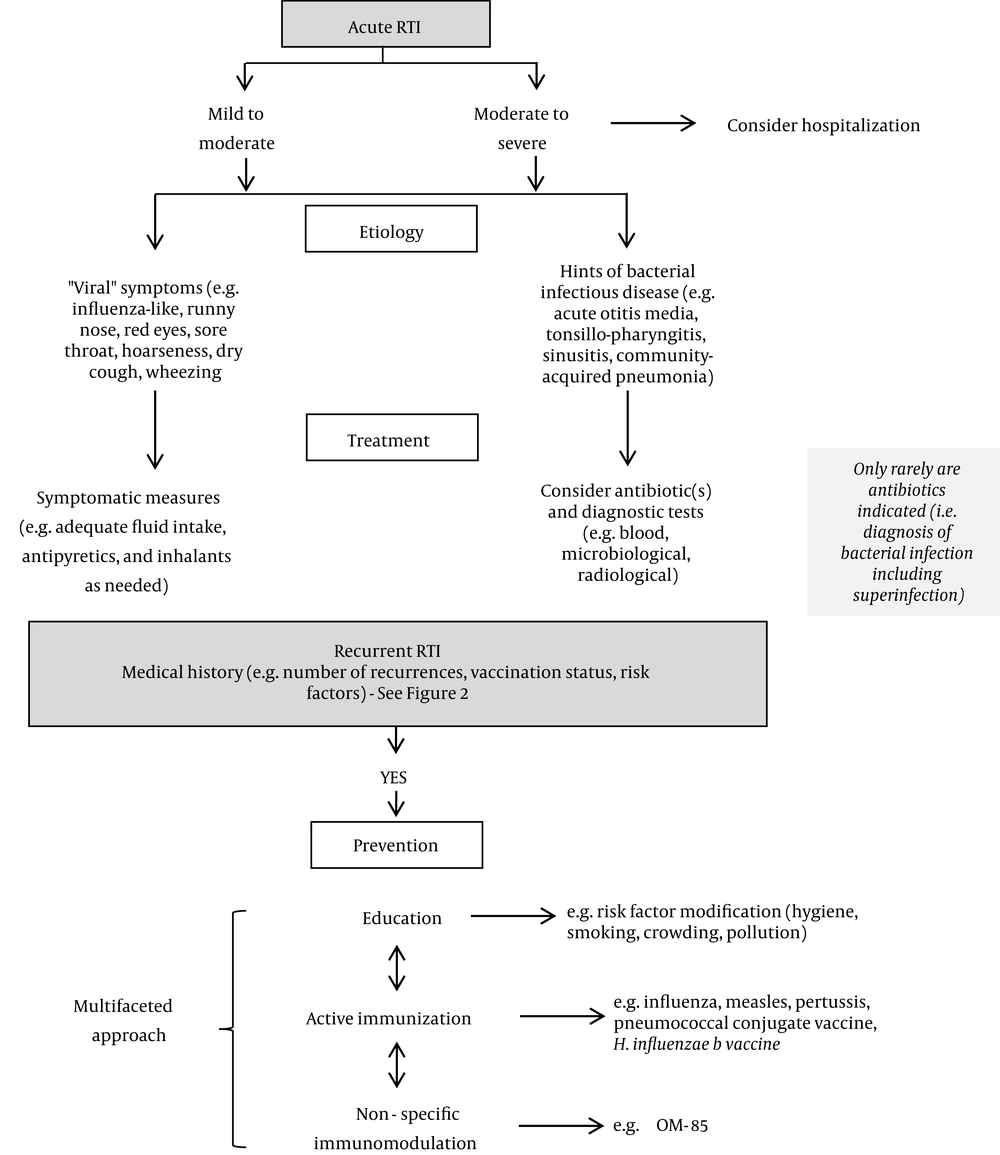
Recurrent infections of the respiratory tissues may result in virus-induced immune dysfunction and can lead to a vicious cycle of recurrent RTIs with bacterial super-infections, exacerbation of cough, and increased risk of asthma development (56-58). In preschool-aged children, RTIs are also a main trigger of recurrent wheezing attacks (49). Wheezing attacks are defined as an episode of progressively increasing shortness of breath, cough, wheezing, chest retraction (or a combination of these symptoms) lasting a minimum of six hours in an individual with a normal chest radiographic examination (56). Furthermore, amongst viral wheezing illnesses in infancy and early childhood, those caused by rhinovirus infections are the most significant predictors of subsequent asthma development in later childhood (59). The widespread use of antibiotics for the treatment of RTIs is associated with adverse effects for the patient and a growing rate of clinical failure due to the rise of antibiotic resistance (25-27). Thus, there is an urgent medical need for a multi-faceted management strategy for recurrent RTIs that not only ameliorates the clinical burden, but also breaks the vicious cycle of viral and bacterial mucosal colonization, inflammation, and defective immune response.
1.6. Improved Management of Recurrent RTIs: A Practical Guide for Physicians
1.6.1. Parental Education and Awareness
Raising awareness among parents of the modifiable risk factors for recurrent RTIs can play a large role in prevention (29). Parents, but in particular mothers, should be educated on the benefits of breastfeeding. A child’s exposure to passive smoking and indoor/outdoor pollution are other risk factors within parental control. Furthermore, it is important to inform parents that surgical approaches (including tonsillectomy and adenoidectomy) are not appropriate solutions for reducing recurrent RTIs for the majority of children, as they may confer only modest benefits that need to be weighed against the risks and potential complications (60, 61).
1.6.2. Active Immunizations
The ultimate goal of vaccination is to provide effective, active immunization against a specific pathogen. Vaccines exist for the common influenza viruses, measles, pertussis (Bordetella pertussis), the potentially invasive bacteria Haemophilus influenzae type b, and many serotypes of Streptococcus pneumoniae. These effective and well-tolerated vaccines are widely used for the prevention of these diseases.
Routine influenza vaccination is recommended for anyone older than six months of age who is without contra-indications, including children and adolescents (although the specific recommendations may vary between countries) (62). Ideally, the vaccination should be performed before the onset of influenza within the community (in the autumn months, if possible), and vaccinations should be offered as long as influenza viruses are circulating (63). Children between six months and eight years of age require two doses, and should receive their first dose as soon as possible after the vaccine is made available. The second dose should be given at least four weeks later (63). The influenza vaccine would be highly recommended for Fred, provided that he does not have any of the characteristics listed as contra-indications (such as previous allergic reaction to the vaccine or its excipients, egg allergy, wheezing, or asthma). A more complete list of contra-indications is reviewed elsewhere (63, 64).
There are several types of influenza vaccines. The oldest are based on inactivated viruses designed for intramuscular use and have been around for more than 50 years (65). Both the live attenuated influenza vaccine (LAIV) and inactivated influenza vaccine (IIV) have been proven effective in children (63). Widespread administration of the vaccine in school-aged children in advance of an epidemic results not only in cross-protection for recipients, but also in indirect (herd) protection for the community (66). Unfortunately, the immunogenicity and efficacy of conventional IIVs have not been completely satisfactory in younger children, and more effective preparations such as LAIV or inactivated adjuvanted influenza vaccines are not yet licensed for use in all countries (65, 67, 68). Moreover, frequent mutations in key viral proteins decrease the efficacy of the annual influenza vaccine and augment viral resistance against standard antiviral agents (69). Finally, the major limiting factor for vaccines is the presence of hundreds of different viral serotypes, rendering it impossible to create a vaccine for each individual pathogen (70). Therefore, although routine influenza vaccination should be recommended, there remains a need for other strategies to limit RTIs particularly those caused by pathogens not covered by vaccines.
1.6.3. Alternative Strategies for Prevention of RTIs: Immunostimulation/Immunomodulation
For recurrent RTIs, preventive strategies are a cornerstone of clinical management, as these provide a means to interrupt the vicious cycle of microbial infection, repeated mucosal inflammation due to the interaction between microbes and the host’s first-line immune defense, and defective immune response (71, 72). Immunostimulants (also known as immunomodulators or immune enhancers) are effective complementary measures that can be used alongside vaccines to enhance or modulate the patient’s innate immune response. By preventing further infection, immunostimulants have beneficial effects on long-term patient outcomes (71, 72). Unlike vaccines, which stimulate immunity against a specific antigen, they act non-specifically to induce a “pre-alert” state in the host and to augment the general immune response. Due to the ability of some immunostimulants to trigger immune responses against respiratory pathogens, they are particularly suitable for children (for whom these immune functions are still maturing) (71, 72).
There are several different types of immunostimulants available, including immunomodulatory bacterial lysates (i.e., OM-85), herbal extracts (i.e., echinacea and garlic), thymic extracts (i.e., thymomodulin), and synthetic compounds (i.e., pidotimod) (73). The use of bacterial lysates (immunomodulators) to enhance non-specific immunity is shifting to the forefront of preventive strategies for recurrent RTIs. Bacterial lysate immunostimulants were first introduced in the 1980s and are the most widely studied. Although the mechanisms of action of other types of immunostimulants are poorly understood, there is evidence that bacterial lysates activate immune cells via pattern recognition receptors (PRR) such as Toll-like receptors (TLRs), which play a key role in pathogen recognition (74-76). PRRs bind to pathogen-associated molecular patterns (PAMPs) that are characteristic of many bacteria, resulting in stimulation of the innate immune response. This triggers activation of antigen-presenting cells (APCs), the production of cytokines and chemokines, increased phagocytic cell recruitment, and the eventual stimulation of adaptive immunity (such as T-cell and polyclonal B-cell maturation and antibody production) (77, 78). In this manner, ingestion of the appropriate bacterial lysates can stimulate the innate and adaptive immune responses and can enhance the natural process of immune system maturation.
1.6.4. How Can We Select the Appropriate/Immunomodulator?
The broad array of available immunostimulants makes it difficult for physicians to select the appropriate agent. An additional difficulty is that not all immunostimulants are supported by robust clinical data demonstrating efficacy and safety in the pediatric population. When faced with such a choice, the use of systematic reviews and meta-analyses provide a useful framework to guide clinical decision-making.
Cochrane meta-analyses have assessed the safety and efficacy of immunostimulants in preventing acute RTIs in children (first published in 2006 and recently updated in 2012) (73, 79). The results of these analyses indicated that compared to placebo, the use of certain agents could reduce the incidence of acute RTIs in susceptible children. The ones that were evaluated in the Cochrane reviews included bacterial products, herbal extracts (echinacea and garlic), synthetic compounds, and thymomodulin. The meta-analyses called attention to the fact that the overall quality of clinical trials was generally poor and that results were subject to high statistical heterogeneity (73). One exception however, was the bacterial lysate immunomodulator OM-85, the agent supported by the most high-quality (A-grade) clinical studies as judged by the Cochrane criteria (documentation of randomization, blinding, efficacy, and safety, including follow-up) (73). When pooling the results of the nine assessed OM-85 studies (four of these nine trials were deemed to be of grade-A quality), the Cochrane review reported a mean reduction in the number of acute RTIs of -1.20 [95% CI:-1.75, -0.66] and a percentage difference in acute RTIs of -35.9% [95% CI: -49.46, -22.35] when compared to placebo (73). The conclusions from the Cochrane analyses have been supported by other independent meta-analyses (33, 80). A systematic review of eight double-blind, placebo-controlled trials with OM-85 showed that treatment with OM-85 significantly reduced the incidence of RTIs in children suffering from recurrent RTIs (33). Of the population of children receiving OM-85, 32% experienced recurrent RTIs as compared to 58.2% in the placebo population (P < 0.001). Importantly, the efficacy of OM-85 appeared to be higher in a sub-population of patients with the highest number of RTIs in the year prior to starting the study. These findings are supported by several other clinical studies, indicating increased clinical benefit with the use of OM-85 in patients at higher risk of recurrent RTIs (33, 81-84).
1.6.5. Is Fred a Good Candidate for Prevention of Recurrent RTIs With an Oral Bacterial Immunomodulator?
Due to his genetic predisposition (family history of atopy), Fred himself is at risk of atopy and asthma. Additional factors such as his preterm birth, young age, and attendance at kindergarten renders him vulnerable to RTIs. It is important, therefore, to minimize Fred’s chances of RTIs, particularly those caused by viruses associated with wheezing attacks and increased risk of developing asthma in later childhood (59). Razi et al. (49) investigated the effects of OM-85 on wheezing attacks in children aged one to six years who experienced three or more acute wheezing attacks induced by RTIs in the previous six months. Children who received OM-85 had significantly fewer wheezing attacks (a 37.9% reduction in the OM-85 group as compared to the placebo group; P < 0.001), a reduced number of RTIs (a 31.4% reduction in mean incidence; P < 0.001), and a reduced incidence of nasopharyngitis (a 37.5% reduction in mean incidence; P < 0.001) over the 12-month study period. In addition, the authors observed that the beneficial effects of OM-85 were maintained for nine months after ending treatment (49).
Fred’s clinical characteristics resemble those of the study population in a randomized, placebo-controlled study testing the effects of OM-85 in children with recurrent upper RTIs (81). The 232 children enrolled in the study were between three and eight years old and had a history of recurrent RTIs. Treatment with OM-85 resulted in a 16% reduction in RTIs over a six-month period, with the greatest treatment effect seen in children who experienced three or more upper RTIs during the study period (81). The efficacy of OM-85 in reducing the incidence of RTIs has been demonstrated independently in several at-risk pediatric populations, including those exposed to pollution, (55) those living in an orphanage (83) and those with Ig subclass deficiency (in whom OM-85 does not affect clinical or laboratory markers of autoimmunity) (85). Altogether, these findings indicate that OM-85 has a good safety profile and is well-tolerated by the pediatric population. Though there is no formal contra-indication, as a general precaution OM-85 should not be administered to children younger than six months of age, since there is not sufficient treatment data for this sub-population.
The timing of the administration of OM-85 coincides with the timing of the influenza vaccine, and therefore one concern is whether the co-administration of OM-85 affects the efficacy and safety of the influenza vaccine. Esposito et al. tested the effects of OM-85 on the humoral and cellular responses against the IIV in 68 children who experienced recurrent RTIs (86). Results from this study showed that the use of OM-85 had no effect on humoral or cell-mediated immunity against the IIV. A secondary finding was that the group who received OM-85 in addition to the IIV had significantly reduced respiratory morbidity compared to the group that received the IIV alone. The OM-85 plus IIV group also had a significantly reduced consumption of antibiotics for RTIs and significantly reduced school absenteeism (86).
Given Fred’s clinical characteristics, the available evidence supports the use of the IIV alongside oral bacterial lysates such as OM-85 as part of a strategy for limiting the occurrence of his RTIs. The preventive effects of immunomodulators such as OM-85 may have a positive impact not only on quality of life for Fred and his mother, but also on the costs of healthcare and indirect societal costs (e.g., his mother’s absenteeism from work) (87). Furthermore, immunomodulators such as OM-85 are given orally and are therefore easy to administer to children. A management algorithm for pediatric RTIs is given in Figure 3.
2. Conclusions
Pediatric RTIs are a clinical and economic burden for patients and their families around the world. Respiratory infections tend to recur and are associated with significant morbidity and mortality, particularly in young children. For treating physicians, recurrent RTIs pose a diagnostic and therapeutic challenge. In light of growing antibiotic resistance and limited efficacy of antibiotics as a long-term treatment option for patients with recurring RTIs, physicians have few options available to them. Preventive strategies are moving to the forefront as part of a holistic effort to minimize the incidence of RTIs and limit their sequelae. Well-established immunomodulators such OM-85 could play a key role in augmenting the limited armamentarium for physicians regularly facing the problem of recurrent pediatric respiratory infections. Though OM-85 has been licensed for use since 1987 and is supported by a large body of clinical research, recent evidence highlights the potential of this agent to be used not only for prevention of respiratory infections but also as an immunomodulator in at-risk populations (33, 81-84).