1. Context
Visceral leishmaniasis, also known as “kala-azar,” or “black sickness,” is one of the major health problems in certain parts of the world. VL becomes a significant opportunistic parasitic disease in people with acquired immunodeficiency syndrome (AIDS), which may be accompanied by treatment failure and relapse in these populations. Control of leishmaniasis is based on reservoir and sandfly control measures, early diagnosis, and adequate treatment. Currently, no vaccine is available for prevention of leishmaniasis.
2. Evidence Acquisition
In this narrative review, last published sources of information on visceral leishmaniasis consisting of books and articles have been reviewed.
3. Results
3.1. Epidemiology
Leishmania species are members of the family Trypanosomatidae, order Kinetoplastida Leishmania species are obligate intracellular parasites of mononuclear phagocytes which establish chronic intracellular parasitism and survive for an infected person’s lifetime (1). Leishmaniasis is a vector-borne zoonotic disease with animal reservoir, such as dogs or rodents (common reservoir hosts), and human beings infected as incidental hosts. Hares also are suspected as a potential reservoir host responsible for the outbreak of leishmaniasis in certain parts of Europe (2). Approximately 21 of 30 species that infect mammals infect humans. Leishmania parasites transmit commonly by the bite of an infected female sandfly. About 70 species of sand flies that belong to the genus Phlebotomus in the Old world (i.e., Europe, Asia, and Africa) and Lutzomyia in the New world (i.e., the Americas) (3) can transmit Leishmania.
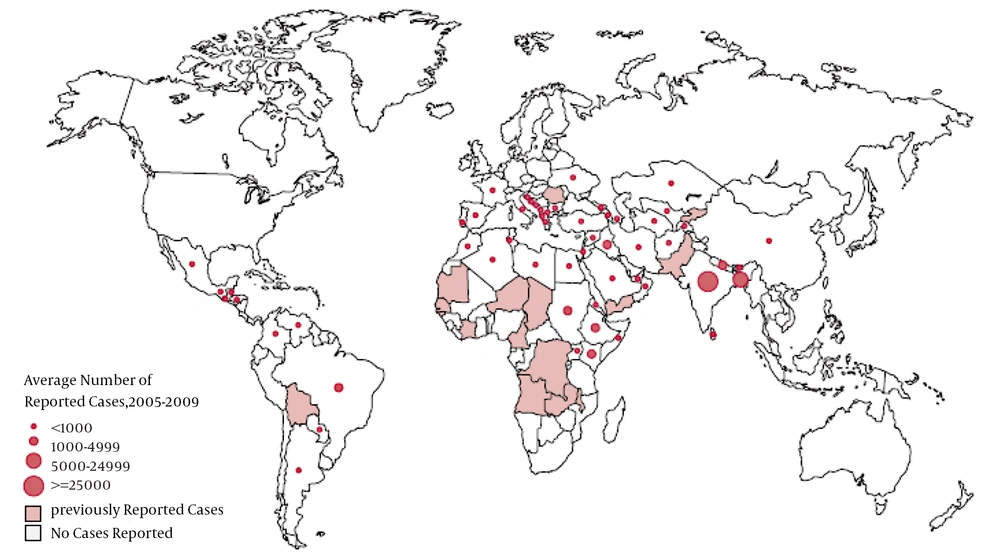
Leishmaniasis mostly occurs in tropical and temperate regions where sand flies are more often found. Disease is endemic in 88 countries (Figures 1 and 2) (4). Annually, about 500,000 new cases of VL and 1 to 1.5 million new cases of cutaneous leishmaniasis occur (5). The estimated incidence of cutaneous and visceral leishmaniasis in Iran is about 42.2 and 0.3 in 100,000, respectively (6).
Distribution of Visceral Leishmaniasis (VL) in Europe, Asia, and Africa (7)
Distribution of Visceral Leishmaniasis Worldwide (8)
Co-infection of leishmaniasis and HIV/AIDS is becoming an important public health problem in different parts of the world (4, 9, 10) and also in our country (11). Travelers to tropical and subtropical areas (including Mediterranean countries) (8) are at risk of exposure to leishmaniasis, and this parasitic infection should be considered in patients with signs and symptoms of leishmaniasis (e.g., fever with hepatosplenomegaly) and a history of travel to these areas, even up to several months or years before onset of clinical symptoms (12).
In endemic areas, children less than 15 years old are commonly affected. In sporadic and epidemic cases of VL, people of all age groups are susceptible, with males at least twice as likely to contract the disease as females.
3.2. Etiology
VL is caused by the Leishmania donovani complex, Leishmania infantum, and Leishmania donovani archibaldi in the Old World and Leishmania chagasi in the New World. Leishmania tropica also may be a causative agent in some parts of the world. Leishmania tropica was first reported in Iran in 2006, and other reports occurred thereafter (13, 14). Leishmania donovani and Leishmania infantum also can cause cutaneous leishmaniasis.
3.3. Pathogenesis
VL is a disease of the mononuclear phagocytic system. Spleen, liver, lymph nodes, and bone marrow are commonly involved. The presence of amastigotes within macrophages is the hallmark of Leishmania infection. Amastigotes are protected from lytic enzymes by their dense surface coat. Interleukin (IL)-12 and interferon (IFN)-γ production by T cells is required for control of the parasites (3, 15). Increased production of other cytokines and chemokines such as IL-1, IL-6, IL-8, IL-15, and tumor necrosis factor (TNF)-α in the spleen and bone marrow also have been shown. IL-10 secreted from T cells has suppressive effects on immune function, and these effects are augmented by macrophages and other cells along with progression of disease that further disturbs immune status (3, 15).
Early production of IL-12 and IFN-γ drives the differentiation of T cells to a protective Th1 phenotype. In general, a Th1 response helps eradicate intracellular microorganisms such as Leishmania (16). Some researchers suggest that normalization of the plasma level of IFN-γ is a reliable index of cure (15). Complement systems are also involved in the killing of parasites. Activation of alternative pathways in the early stages of infection and the classical pathway in the chronic stage of disease could be addressed by fluctuation of C3 levels in different stages of the disease (17). Production of auto antibodies also has been shown in VL patients (18). Suggested mechanisms of innate and acquired immunity to Leishmania are shown in Box 1 (16). The natural killer (NK)-cell-activated macrophage system is central to the innate response to Leishmania.
| Mechanisms |
|---|
| Innate immune response |
| Complement-mediated lysis |
| IL-12-dependent NK-cell production of IFN-?, leading to macrophage activation |
| Activated polymorphonuclear leukocytes |
| Interferon-a/ß activation of macrophages |
| Toll-like receptor signaling |
| Acquired immune response |
| Class I-restricted cytotoxic CD8 T cells |
Leishmania relies on a wide array of mechanisms of pathogenesis to evade the host immune response. Proposed mechanisms by which Leishmania could evade immune systems are summarized in Box 2 (16).
| Mechanisms |
|---|
| Resistance to complement-mediated lysis |
| Impaired macrophage microbicidal function |
| Impaired antigen presentation to T cells |
| Synthesis of immunosuppressive mediators (e.g. IL-10, TGF-ß, PGE2) |
| Generalized depression of T-cell responses |
| Expansion of regulatory T-cell population |
3.4. Life Cycle
The Leishmania parasites have two different morphologic stages. Promastigote is the flagellated form found in sand flies and culture, and amastigote is a non-flagellated tissue form (2 - 4 mm in diameter) that replicates in macrophage phagosomes in mammalian hosts (1). Lutzomyia or Phlebotomus sand flies bite the mammalian host (e.g., rodents and canines), taking up amastigotes. Amastigotes convert to metacyclic promastigotes in the gut of the sandfly in 4 to 14 or more days. After that, if a Lutzomyia or Phlebotomus sandfly bites a mammalian host, metacyclic promastigotes are inoculated into the host’s skin. Promastigotes generated after inoculation enter mononuclear phagocytes in the skin. Finally, amastigotes develop and multiply in mammalian mononuclear phagocytes to cause asymptomatic self-resolving infection, or one of three types of leishmaniasis (1, 7, 19).
3.5. Incubation Period
Incubation period after vector transmission ranges typically from six weeks to six months (two to six months) but may have a range as wide as ten days to more than ten years. Typical symptoms of the disease developed from four weeks to 18 months (mean: 8.5 months) after birth in congenital transmission (19). Shorter duration has been suggested for those who are immunocompromised (such as HIV patients) and those who were infected by non-vector transmission such as contaminated blood transfusion (20).
3.6. Infection Risk Factors
Residence close to focus of reservoirs, lack of tap water, dams and water irrigation scheme, absence of sewer and electricity, house with mud floor or walls, open garbage containers, animals in or near the house, and living with a case in the household are known risk factors for acquisition of leishmaniasis (21-23). Shared needles used by intravenous drug users are a direct means of acquisition of leishmaniasis as a non-vector transmission, specifically in HIV patients (24).
3.7. Modes of Transmission
Vector borne transmission occurs with the bite of an infected female phlebotomine sandfly. The sandfly becomes infected by taking blood meal from an infected mammalian host. A total of about 30 species in the Phlebotomus genus (Old World) and the Lutzomyia genus (New World) have been identified as vectors. Sand flies are relatively weak, noiseless fliers. They rest in dark, moist places, and are typically most active in the evening and night hours.
Non-vector transmission includes blood transfusions (Leishmania tropica has been proven to survive for at least 25 days in blood products stored under standard conditions), contaminated needles of drug users, organ transplants, laboratory infection, and congenital transmission (i.e., blood exchange from the mother to the child during labor and transplacental infection during pregnancy). In a study in the endemic part of Iran, investigation of L. infantum K-DNA by PCR-ELISA showed that newborn infants of asymptomatic pregnant women are not at greater risk for transplacental infection (25, 26).
3.8. Clinical Manifestation
This parasitic disease may have three clinical manifestations that include cutaneous leishmaniasis, mucocutaneous leishmaniasis, and visceral leishmaniasis. In some sources, diffuse cutaneous leishmaniasis (DCL) is considered as a distinct entity (4). Despite these different clinical manifestations, infection also may be asymptomatic. Asymptomatic state to clinically symptomatic ratio varies according to geographic area and ranges from 1:2.6 in the Sudan to 18:1 in Brazil (27). The reported ratio in Iran is 13:1 (28).
VL caused by all species has five characteristic hallmark features that include organomegaly (e.g., massive splenomegaly and moderate hepatomegaly), fever, cachexia and weight loss, pancytopenia and hypergammaglobulinemia. Overall, the most common clinical features are anemia, fever, and splenomegaly (26, 29). The onset of disease can be insidious or acute. Skin manifestations in VL are frequent. The fever pattern is distinctive; it rises two to four times per day. Fever can rise up to 41°C with a pattern of double daily spikes. However, this pattern is rarely seen.
“Kala-azar” means “black sickness” and refers to the earth-gray skin color that is common in infected individuals, especially in India. Diffuse nodular skin lesions may also be present. Mucosal lesions consisting of oral or nasal ulcers may accompany the systemic illness, particularly in patients in the Sudan. Thrombocytopenia results in petechiae, ecchymoses, and gingival bleeding. With disease progression, diarrhea, malabsorption, hypoalbuminemia, peripheral edema, cachexia, and debilitation could develop. Immune compromise secondary to neutropenia may cause bacterial super-infections. The spleen usually is massively enlarged (i.e., soft and non-tender). The liver also may enlarge (i.e., sharp edge, soft consistency, and a smooth surface) (30). Lymphadenopathy is common in the Sudan (31).
In a study in Iran, the majority of the infected children (80%) were from rural areas and nomads. The disease was commonly observed during the months of March through May. The most common symptom was fever. Fifty-seven percent of the patients had abdominal distension, and 49% had anorexia. Fever and splenomegaly were present in more than 95% of the patients. Hepatomegaly was seen in 76% and edema in 10% of the cases, but palpable lymph nodes were found in only 3.7% of the children (Table 1) (30).
| Symptoms | Patients, No. (%) | Signs | Patients, No. (%) |
|---|---|---|---|
| Fever | 205 (95) | Splenomegaly | 214 (99.5) |
| Abdominal distension | 122 (57) | Fever | 205 (95.3) |
| Anorexia | 105 (49) | Hepatomegaly | 164 (76.2) |
| Sweating | 95 (44) | Edema | 23 (10.6) |
| Pallor | 81 (38) | Jaundice | 12 (5.5) |
| Weight loss | 74 (34) | Sparse and brittle hairs | 10 (4.6) |
| Malaise | 71 (33) | Lymphadenopathy | 8 (3.7) |
| Cough | 67 (31) | Petechiae, Purpura and echymosis | 6 (2.7) |
| Vomiting | 49 (23) | ||
| Chills | 41 (19) |
aReference: (23).
Visceral leishmaniasis can be complicated by serious secondary bacterial infections such as pneumonia, dysentery, and pulmonary tuberculosis (32), which may contribute to increased morbidity and mortality. Staphylococcus aureus, Klebsialla pneumonia, and Pseudomonas aeruginosa are common pathogens that cause septicemia (33).
Lung (i.e., pneumonitis, septal fibrosis, pleural effusion, and mediastinal adenopathy) and gastrointestinal tract (i.e., diarrhea) involvement are unusual clinical presentations of leishmaniasis reported in patients co-infected with HIV (34). Leishmaniasis also may complicate lung transplant recipients (35). Clinical manifestations of VL in HIV-infected patients (without severe immunosuppression) are similar in non-HIV infected individuals, although some differences have been reported, such as lower rate of splenomegaly and involvement of atypical sites (23, 36). Interestingly, immune reconstitution inflammatory syndrome (IRIS) is a rare occurrence in HIV/VL co-infected patients (36).
3.9. Diagnosis
3.9.1. Hematological Examination
The laboratory findings in VL include anemia, leukopenia, thrombocytopenia, and hypergammaglobulinemia. Anemia is almost always present and may be severe. It is usually normocytic and normochromic. It appears to be due to a combination of factors including hemolysis, marrow replacement with Leishmania-infected macrophages, hemorrhage, splenic sequestration of erythrocytes, hemodilution, and marrow suppressive effects of cytokines such as TNF-α. Leukopenia is also prominent, with white blood cell counts occasionally as low as 1000/mm3. It is not known whether the observed neutropenia is due to increased margination, splenic sequestration, or an autoimmune process, or a combination of these factors. Eosinopenia, or absence of eosinophils, is frequently observed. Of note, anemia and neutropenia have not been prominent in patients with VL who have undergone splenectomy (30).
3.9.2. Detection of Parasite
The identification of parasite amastigotes in tissue smears or culture has been the recommended method of VL diagnosis. Aspirates of the spleen (98% positive), sternal bone marrow (85% positive), and liver (60% positive) could reveal the tissue form of the parasite. In a study in Iran, 89.5% of the splenic smears were found to be positive for Leishman bodies. Spleen puncture may cause severe hemorrhage and hypotension and is generally not recommended except in certain circumstances (37). Leishmania amastigotes also can be found in bronchoalveolar lavage (BAL) fluid in immunocompromised hosts (38). To ensure that the visualized structures are amastigotes rather than other “dot-like” organisms (e.g., Histoplasma spp.), an experienced observer should look for the characteristic size (2 - 4 mm in diameter), shape (round to oval), and internal organelles (the nucleus and kinetoplast). In particular, the kinetoplast should be visualized: it is a rod-shaped, specialized mitochondrial structure that contains extranuclear DNA.
Parasites were identified more rapidly in children younger than three years old compared with those greater than three years old. Culture for leishmaniasis is usually performed in NNN (Novy, MacNeal, Nicolle) or Schneider’s medium (30, 39, 40). Microscopic examination of peripheral blood smears is recommended primarily in HIV-infected patients with VL (41), but recent reports also recommend this method in non-HIV patients (42).
3.9.3. Serologic Tests
High parasite-specific antibody titers are frequently observed in patients with active VL, which can be used as supportive data in the diagnosis. Serologic tests alone are neither sufficiently sensitive nor specific to definitively confirm or exclude leishmaniasis. Serology may be positive in individuals from endemic areas because of past exposure and may not be indicative of current active disease. While immunocompetent patients with VL usually have a high titer of antibodies, immunocompromised patients often do not have detectable parasite-specific antibodies; thus, serology may not be a sensitive test in these hosts. Individuals with viscerotropic disease due to L. tropica also have low or undetectable titers of antibodies. Serologic tests using enzyme linked immunosorbent assay (ELISA), complement fixation test, indirect immunofluorescence (IFA), and direct agglutination tests (DAT) are available.
A. Enzyme linked immunosorbent assay (ELISA): Soluble antigen or sonicated extract of promastigotes is used to capture antibodies specific to Leishmania. The ELISA test is 100% specific with sensitivity above 98%.
B. K39 ELISA: This assay uses the recombinant K39 protein as the antigen in ELISA assays to detect antibodies in the serum of patients with VL and has been shown to be more specific in active visceral disease. The K39 ELISA test is approximately 98% specific with sensitivity more than 95%. In a study by Alborzi et al., the sensitivity and the specificity of anti-K39 strip-tests were 82.4% and 100%, respectively. This report confirms the usefulness of the rK39 strip test in Iran and other developing countries for the diagnosis of VL in infants (43). Tests could be conducted simply by taking a drop of blood without the need for any laboratory facilities, which makes it more popular among clinicians.
C. Direct agglutination test (DAT): The test is based on antigen-antibody reaction. Trypsin-treated, strained, and formalin-preserved promastigotes are used as antigens, which show agglutination with specific antibodies present in the patient’s serum. Compared with the results obtained from smears, the sensitivity and specificity of the DATs were 94% and 72%, respectively.
D. Complement fixation test: The complement fixation test is used to detect specific antibodies present in the serum. The antigen used in the test is prepared from either human tubercle bacilli (Witebsky, Kligenstein & Kuhn: WKK antigen) or from Kedrowsky’s acid test bacillus. Cross reactions are observed in cases of pulmonary tuberculosis and leprosy.
E. Immunoflourescent antibody test (IFA): The parasite antigen labeled with fluorescent dye is conjugated with serum antibodies and seen under a fluorescent microscope. The test could be positive in the very early stages of infection and undetectable six to nine months after cure. If the antibodies persist in low titers, it is a good indication of a probable relapse (30, 40).
3.9.4. Skin Test
The leishmanin skin test (LST), also known as the Montenegro test, is an intradermal injection of a suspension of killed promastigotes. It measures delayed hypersensitivity reactions and appears to be the only screening test capable of detecting asymptomatic Leishmania infections. A positive LST result is thought to indicate durable cell-mediated immunity after asymptomatic infection or clinical cure of VL (19, 30). LST positivity has direct correlation with age, and the highest incidence of positive test results has been shown in higher age groups of asymptomatic patients (44).
3.9.5. Formol Gel Test
The concentration of gamma globulins in blood increases considerably following infection. Formaldehyde has a tendency to bind these serum immunoglobulins. The aldehyde test procedure is simple. Opaque jellification, like the white of a boiled egg, indicates a positive reaction. Jellification time indicates intensity of infection (i.e., if jellification occurs within two minutes, it indicates the greatest intensity of reaction) (30).
3.9.6. Rapid Diagnostic Test
Rapid dipstick tests based on the recombinant K39 protein are designed for ease and rapid diagnosis of kala-azar in the field. This antigen is an epitope apparently conserved on the amastigotes of the Leishmania species. These ELISA testing detects circulating anti-K39 (IgG). The rK39 ELISA test is 97 - 100% specific with sensitivity of 98 - 100% (30, 43).
3.9.7. Molecular Techniques
Molecular probes, Leishmania-nested polymerase chain reaction (LnPCR) analysis, and quantitative PCR improved sensitivity and specificity. These highly sensitive and specific diagnostic tools are increasingly used for diagnosis of VL. The ability for utilization of a wide range of clinical samples such as tissue aspirates, blood, urine, and even buccal swabs makes these tests more advantageous (7, 19, 30). Recently, some researchers have questioned the reliability of these tests in comparison with other traditional tests such as the LST and the immunofluorescent antibody test for diagnosis of active VL in endemic areas (44).
Parasite DNA load also may be an aid in the management and surveillance of the response to treatment (45). The kinetoplast DNA (kDNA) polymerase chain reaction (PCR)-ELISA method may play a role in the detection of asymptomatic patients in conjunction with serology and LST (44). According to a study by Alborzi et al., a positive PCR test together with IFA ≥ 1:80 could differentiate acute infection from previous exposure. Combination of these tests improves sensitivity of the PCR test (26). Molecular tests are highly sensitive and specific for the diagnosis of VL in HIV-infected patients (36).
3.9.8. Other Tests
Among the most recently described methods, the western blot technique is highly sensitive and specific and provides broader information about the parasite’s antigenic profile. 65-kDa antigenic component was recognized by 100% of serum specimens from patients with clinically and parasitologically confirmed VL. It was never identified in the control sera tested (100% specificity) (46).
Matrix-assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF) is a promising method for identification of Leishmania at the species level with sensitivity comparable to molecular (PCR) methods but in a much shorter time (47).
3.10. Prevention and Control
VL control could be achieved by interrupting the transmission cycle of the parasite, applying control strategies targeted to the vectors and reservoirs (i.e., killing of infected dogs and use of insecticides), or a combination of these methods. Travelers to endemic areas should protect themselves from sandfly bites. Currently, no vaccine for humans is available (27). Three vaccines are registered for use in dogs: two in Brazil and one in Europe. Their impact on the transmission to humans in zoonotic VL has not yet been firmly established.
Case detection and management also play an important role in control of disease transmission in anthroponotic VL areas. Untreated VL patients can act as a reservoir of parasites and contribute to disease transmission in these areas (such as in L. donovani endemic regions in the Indian subcontinent and in East Africa) (3, 30). Secondary prophylaxis with anti-leishmanials is likely to be efficient in patients co-infected with HIV and VL. According to WHO recommendations (for avoidance of resistance development) anti-leishmanial agents should be switched in secondary prophylaxis to drugs not given in treating primary VL cases (48-50).
3.11. Management and Treatment
Although there are nearly 25 compounds having anti-leishmanial effects, only a few are used for humans, and most of these are parenteral. The pentavalent antimony compounds have remained a mainstay for nearly 80 years. Pentavalent antimonials are sodium stibogluconate (Pentostam) and meglumine antimoniate (Glucantime). The products differ by the strength of the SbV solution: meglumine antimoniate solution contains 8.1% of Sbv (81 mg SbV/mL), and sodium stibogluconate contains 10% Sbv (100 mg SbV/mL). Antimonials act by their toxic effect on the parasite after reduction of SbV to SbIII (3). In a randomized clinical trial by Dr. Alborzi et al., a short course of Glucantime was recommended for treatment of VL in southern Iran. In this approach, treatment had been continued for a week after defervescence and was found to be adequate (51).
Amphotericin B is an antifungal agent with anti-leishmanial activity. It binds irreversibly to ergosterols in the membrane of the Leishmania parasite, causing pores that leak ions and subsequent cell death. Amphotericin B kills extracellular and intracellular forms of Leishmania. Experts recommend amphotericin B in every patient with jaundice, huge splenomegaly, ill and toxic patient with DIC (51). The incorporation of amphotericin B into phospholipid vesicles (liposomes) or cholesterol esters facilitates the uptake of the drug by the macrophages (i.e., where the parasites live). The drug also can be targeted to particular sites of infection, whereby more drug will be available to interact with the parasite ergosterol and less to react with the human cholesterol, thus reducing its toxic effects (27). Liposomal amphotericin B is the only drug approved by the U.S. food and drug administration (FDA) for the treatment of VL.
A major advance in the treatment of VL has been the development of miltefosine, an orally administered phosphocholine analogue (30). Demonstrated bioavailability ranges from 82% to 94% in animal studies. (Bioavailability calculation in humans is limited because of the inability to intravenously administer.) Because miltefosine can open the epithelial tight junction, it may increase the bioavailability of certain drugs such as protease inhibitors and non-nucleoside reverse transcriptase inhibitors (52). Relapse rates with miltefosine reach up to 20% (53, 54).
In VL cases, the tissue forms of parasites drive out cholesterol from the cell membrane of the macrophage, which results in impaired antigen presentation function. Additionally, signaling of the IFN-γ receptor may be disturbed. Cholesterol liposomal formulation of IFN-γ has shown success in the treatment of drug sensitive and resistant VL (55).
3.12. Management of HIV/VL Co-Infection
Recently, an increase has been reported in the number of geographical areas of HIV and leishmaniasis infection (24). The individual development of each disease may accelerate the onset or progression of the other. Rapid progression of VL, drug interaction, treatment failure, relapse, and increased mortality are known consequences of HIV/VL co-infection. Antiretroviral treatment (ART) started after completion of VL treatment is a more acceptable strategy in HIV/VL co-infected patients (36). Antiretroviral treatment in HIV/VL co-infected patients can lead to PKDL (23). According to WHO recommendations, antimonials should be avoided for treatment of these patients because of the high failure rate and increased toxicity (23, 27, 56). The suggested agent, dose, and duration of treatment is liposomal amphotericin B, 3-5 mg/kg IV (or 40 mg/kg total dose) daily or intermittently for ten doses (i.e., on days 1-5, 10, 17, 24, 31, and 38) (23, 36). Recently, combination regimens of AmBisome (liposomal amphotericin B) with miltefosine have been used successfully in these patients (50).
VL relapses frequently occurred after successful treatment of primary VL in these populations. Without secondary prophylaxis, reported relapse usually occurs in the first six months after primary treatment. Secondary prophylaxis may be achieved with a monthly amphotericin B infusion (57). HIV/VL co-infected patients also are at greater risk for drug toxicity between anti-leishmanial agents and antiretroviral agents. For example, concomitant administration of amphotericin B and zidovudine has the potential risk of myelotoxicity and nephrotoxicity with unknown mechanism (58).
3.13. Emergence of Drug Resistance
The emergence of drug-resistant leishmaniasis is increasing in the world and is estimated to be about 5 - 70% (30).
3.14. Resistance of Antimonials
Decreased drug uptake, increased efflux mechanism, reduced intra-parasite concentration, drug inactivation, and gene amplification are some host factors responsible for the development of resistance to antimonials. The thiol molecule can prevent glutathione formation and reduction of pentavalent antimonials to trivalent formation inside the macrophage. Increase in intracellular thiol levels is also associated with high antimonial resistance (55).
Development of amphotericin B resistance explained with alteration in the membrane composition (i.e., sterols in the cell wall replaced by another precursor) leads to decreased binding and reduced uptake of amphotericin B into the cell. Drug efflux mechanism also may lead to resistance (55).
Gene inactivation (i.e., point mutations in the LdMT gene) is a proposed mechanism that leads to reduced drug uptake and treatment failure with miltefosine. The ATP-binding cassette (ABC) transporter superfamily also can mediate miltefosine resistance via active drug efflux. Lower content of unsaturated phospholipids (e.g., fatty acid and sterol) can reduce the ability of the drug to insert into the external monolayer (52, 55).
Treatment failure may be caused by factors other than direct drug resistance. Species-specific drug tolerance, increased infectivity (i.e., by increased metacyclogenesis at the promastigote level), extra immune suppression (i.e., by over-production of IL-10), reduced exposure to the drug (i.e., by overexpression of host cell multidrug resistance protein 1, [MRP1] that leads to drug efflux), and increased tolerance to the effector molecules of the immune system are proposed mechanisms that may contribute to treatment failure (59).
4. Conclusions
Development of novel drugs and diagnostic tests has allowed us to better manage VL. Although leishmaniasis is one of the oldest known parasitic infectious diseases, increasing prevalence of VL among specific populations, recent reports of disease reactivation and flare-up in clinically asymptomatic patients after the onset of immunosuppressive therapy, the risk of disease acquisition by tourists in endemic areas (e.g., in the Mediterranean), and difficulties in the prevention and control of the disease (i.e., given the diversity and distribution of vectors and reservoirs), leishmaniasis has again attracted the attention of researchers. Treatment failure has been reported in some areas, and drug-resistant VL has become a problematic challenge for clinicians.