1. Introduction
Nijmegen breakage syndrome (NBS, an ataxia telangiectasia variant) is a rare autosomal recessive congenital disorder, causing chromosomal instability, probably as a result of a defect in the double stranded DNA repair mechanism and hypersensitivity to ionizing radiation (1). This syndrome is characterized by a very high predisposition to lymphoid malignancies, short stature, an unusually small head size (microcephaly), distinctive facial features, recurrent respiratory tract infections, increased risk of cancers, intellectual disability and other health problems (2). Some patients with ataxia telangiectasia-like syndromes, like patients with NBS, have immunodeficiency with decreased serum IgA and IgG and increased IgM levels, due to class switch recombination defects, which can be associated with more severe infections (3, 4).
In this report, we present for the first time, a patient with NBS with initial diagnosis of hyper IgM syndrome, who developed splenomegaly and pancytopenia in follow up visits. He was treated with Rituximab, because of the probability of class switch recombination defects, with very good response to treatment.
2. Case Presentation
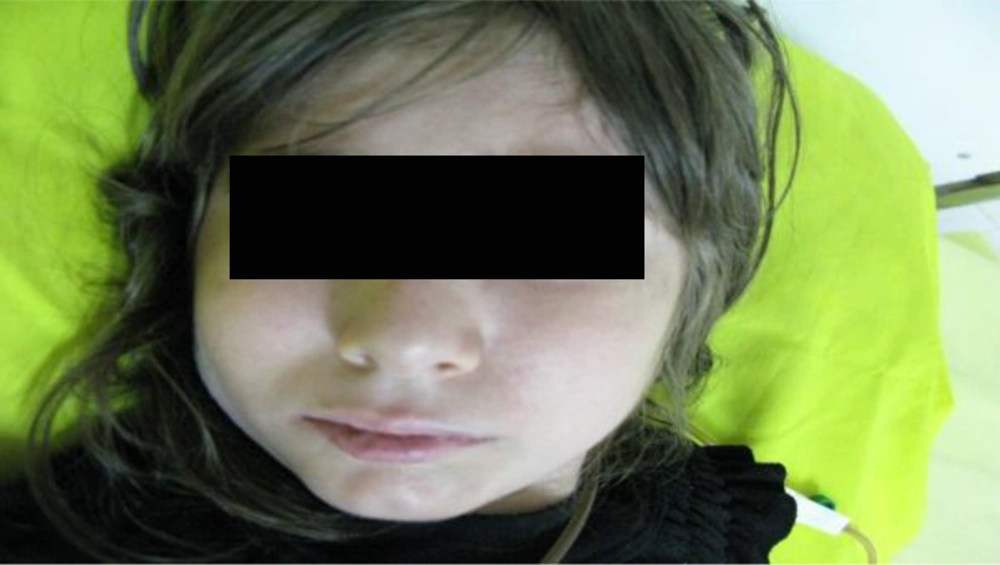
An eight-year-old female was admitted to the hospital because of recurrent infections and failure to thrive. She had microcephaly (head circumference = 42 cm) and typical bird-like facial appearance (Figure 1).
She had a history of several flues and recurrent infections (pneumonia and sinusitis) from the age of four years. Immunologic investigations showed high IgM and low IgA and IgG levels (Table 1). Percentage of T and B lymphocytes and NK cells, evaluated by flow cytometry, are shown in Table 2. According to her special phenotype (microcephaly, bird like face, etc.), her failure to thrive, low IgA and IgG and high IgM levels and exclusion of other primary immunodeficiencies, the patient was diagnosed with Nijmegen breakage syndrome. Genetic counseling also confirmed this diagnosis, according to its inheritance pattern and the patient’s family tree. The patient underwent IVIg therapy monthly and received prophylactic antibiotics. She was doing well for 18 months, when she developed pancytopenia (Table 3) and huge splenomegaly. Fanconi anemia was ruled out. Autoimmune and virological (Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) polymerase chain reaction (PCR)) assessments showed normal results. As NBS patients are predisposed to malignancies, bone marrow aspiration and biopsy was done twice that had a normal pattern, and hematologic malignancies were ruled out. According to hyper IgM pattern and splenomegaly, the probability of class switching recombinant defects was proposed. The patient was treated with Rituximab (Anti CD20 mAb), 375 mg∕m2 weekly for four weeks. Cytopenia was improved after four weeks of therapy with Rituximab (Table 3). The spleen size shrunk to normal and the patient underwent monthly IVIg therapy and prophylactic antibiotic. She was symptom free in her two years follow up.
| Immunoglobulins | Value | Normal Range |
|---|---|---|
| IgM, mg/dL | 1200 | 40 - 150 |
| IgA, mg/dL | 7 | 40 - 300 |
| IgG, mg/dL | 18 | 600 - 1300 |
| IgE, IU/mL | 1 | Up to 115 |
| CD Markers, % | Value | Normal Range |
|---|---|---|
| CD 3 | 82 | 49 - 80 |
| CD 4 | 60.7 | 20 - 60 |
| CD 8 | 22.2 | 10- 37 |
| CD 19 | 2.9 | 3 - 14 |
| CD 16 | 16 | 5 - 19 |
| Before Rituximab Therapy | After Rituximab Therapy | |
|---|---|---|
| White blood cells | 1700 (Neutrophils: 26%, lymphocytes: 74%) | 5300 (Neutrophils: 53%, lymphocytes: 41%) |
| Lymphocytes, cell/µL | 1258 | 2173 |
| Neutrophils, cell/µL | 442 | 2809 |
| Hemoglobin (Hb), g/dL | 7.6 | 15.3 |
| Platelets, cell/µL | 82000 | 205000 |
3. Discussion
Nijmegen breakage syndrome (NBS) (OMIM251260) is a rare autosomal recessive condition. The major manifestations include microcephaly, a distinct facial appearance, growth retardation, recurrent infections due to combined immunodeficiency, spontaneous chromosomal instability, hypersensitivity to ionizing radiation, and a very high predisposition to lymphoid malignancies. The immunological, cytogenetic, and cell biological findings are very similar to those in ataxia telangiectasia (5-7). Defects of class switching are typically associated with low serum IgG and IgA, and normal to increased serum IgM levels, a phenotype reminiscent of hyper IgM syndrome, which may be associated with more severe infections (2).
In this patient with NBS, the presence of hyper IgM phenotype and its associated class-switching defect had resulted in lymphoproliferation (huge splenomegaly) and pancytopenia. Rituximab therapy was started because of its good effect in treatment of hyper IgM syndrome complications. Her splenomegaly and cytopenia disappeared and she was controlled well with Rituximab therapy for four weeks.
Hennig et al. reported two patients with intrinsic B-cell class switch defects (subclass of hyper IgM syndromes), with lymphoproliferation and autoimmunity (such as autoimmune hemolytic anemia, autoimmune thrombocytopenia and lymphoproliferative disorders), that had improved by Rituximab therapy and remained well-controlled during the follow-up period (three to four years) (8).
The patient was diagnosed to have an intrinsic B-cell class switch defect, when lymphoproliferation and cytopenia were detected and responded well to Rituximab therapy.
This case report suggests that class-switching defects should be considered in patients with ataxia telangiectasia variants, especially when they have hyper IgM phenotype, and if they present lymphoproliferation, Rituximab therapy could be an effective treatment.