1. Background
Staphylococcus aureus (S. aureus) has attracted the attention of many researchers as an important bacterium producing beta-lactamases (1). Methicillin was the first semi-synthetic penicillin discovered against this bacterium (2), but methicillin-resistant S. aureus (MRSA) is resistant to all antibiotics with a beta-lactam ring, including penicillins and cephalosporins. MRSA strains are resistant to other antibiotics as well, and they present as a multi-drug resistant (MDR) organism. Among the mechanisms proposed for penicillin and cephalosporin resistance in MRSA, intrinsic resistance mediated by chromosomes is well-known. This mechanism involves the production of low affinity penicillin-binding proteins (PBP; PBP-2a), which can be replaced by high affinity PBPs at antibiotic concentrations that deactivate other potentially lethal PBPs (3).
In antibiotic susceptibility tests, most isolates of S. aureus show heterogeneous phenotypic expression. Few isolates (1 in 104 to 108) express resistance to methicillin spontaneously and are reported as MRSA. In MRSA strains on the borderline of MIC with beta-lactam antibiotics, acquired resistance can be detected in the laboratory by adding inhibitors such as clavulanic acid, which lowers the MIC to a susceptible range. However, most routine laboratories do not have access to such inhibitors (3). The latter resistance mechanism is encountered by changes in the normal PBPs with altered tendencies to antibiotics (4).
Infections due to MRSA are not different from other staphylococcal infections though several strains of MRSA may be more virulent than regular Staphylococci. Identifying infections caused by MRSA requires laboratory testing, such as the antibiotic susceptibility test, because it is more difficult to manage MRSA infections without knowing the antibiotic resistance pattern (5). Thus, awareness of the prevalence of MRSA and methicillin-sensitive S. aureus (MSSA) strains as well as their antibiotic sensitivity patterns is essential for the effective control of therapeutic outcomes. Many laboratories rely on standard institutional recommendations (e.g., the clinical and laboratory standard institute [CLSI] or the European committee on antimicrobial Susceptibility testing [EUCAST]) and use cefoxitin as a single phenotypic marker for reporting methicillin while others use the mecA gene for molecular testing. However, this time-consuming method is unmanageable when testing a single clinical specimen, so it is not offered as a routine test by hospital authorities; thus, many laboratories depend on phenotypic tests. Periodical checks for the methicillin resistance of S. aureus isolates or their sensitivity to other tests are a prerequisite.
2. Objectives
This study focused on the detection of intrinsic and acquired methicillin resistance, including the effects of beta-lactamase inhibitors and the assessment of antibiotic sensitivity patterns for clinical isolates of S. aureus that were sensitive and resistant to methicillin.
3. Methods
For this quantitative study, 105 clinical S. aureus isolates were collected from patients admitted to the university teaching hospitals of Tabriz from April to September, 2015. The sources of S. aureus included blood, wound discharge, urine, and synovial fluid. The isolates were identified to the species level utilizing Gram staining, catalase, coagulase, mannitol fermentation, and DNase production tests (5, 6). Identified S. aureus isolates were finally stored in trypticase soy broth (HiMedia) containing 20% glycerol (Merck) at -70°C.
3.1. Antibiotic Sensitivity Testing
The oxacillin agar screening test was performed for the detection of methicillin resistance in S. aureus isolates as per the CLSI recommendation (7). Briefly, Mueller-Hinton agar (HiMedia) containing 6 µg/mL oxacillin (Sigma) was prepared, and 4% NaCl (Merck) was added to it. A bacterial suspension equivalent to 0.5 McFarland (108 CFU/mL) was prepared from the overnight growth of all test isolates and then diluted 1: 100. Later, 10 µL of the above bacterial suspension was used to inoculate plates harboring oxacillin, which were incubated at 35°C for 24 hours. The growth of a single colony was considered to be oxacillin resistant (8).
The antibiotic susceptibility test was performed by disk agar diffusion and agar dilution methods (7, 9). The antibiotic susceptibility patterns of 105 S. aureus isolates toward 13 antibiotics were studied by the disk agar diffusion method using the following antibiotics (HiMedia): ciprofloxacin (5 μg), rifampin (5 μg), clindamycin (2 μg), cephalothin (30 μg), sulfamethoxazole (25 μg), amikacin (30 μg), erythromycin (15 μg), penicillin (10 U), cephalexin (30 μg), gentamicin (10 μg), lincomycin (2 μg), and chloramphenicol (30 μg). According to the CLSI recommendation, sensitivity against methicillin was determined using a cefoxitin (30 μg) disc, which is considered a surrogate marker for methicillin. Vancomycin susceptibility was checked by the MIC test as per the CLSI guidelines, using an E-test (Liofilchem, Italy), and the breakpoints for resistance were those defined by the CLSI (7). All tests were performed on Mueller-Hinton agar, and the plates were incubated at 35°C for 24 hours. Sodium chloride (NaCl) was not added to the agar medium.
The diameters of the growth-inhibition zones were measured after incubation at 35°C, and the results were interpreted according to CLSI guidelines (7). S. aureus ATCC 25923 was used as a positive control for the antibiotic susceptibility test along with other strains: S. aureus ATCC 29213 and Enterococcus faecalis ATCC 29212. The MIC of oxacillin was determined by the agar dilution method, as recommended by the CLSI (7). The test was performed with and without additions of 2% NaCl to the oxacillin medium in 11 concentrations (ranging from 0.25 - 256 μg/mL) in four series; namely, oxacillin was used with and without 2% NaCl, and oxacillin with 4 μg/mL clavulanic acid was used with and without 2% NaCl. The bacterial inoculum of 104 CFU/mL was used to inoculate plates with different antibiotic concentrations, and the plates were incubated for 20 hours at 37°C. Isolates with an oxacillin MIC < 8 μg/mL with NaCl were defined as “intrinsic resistant.” Isolates with an oxacillin MIC < 2 μg/mL were defined as “sensitive,” and those with MICs between 2 μg/mL and 8 μg/mL were considered “borderline resistant” (10).
Beta-lactamase production was tested by the iodometric method. The MICs of resistant isolates were determined after the addition of the beta-lactamase inhibitor, clavulanic acid (Sigma, ACSC 80201), to the culture medium at a concentration of 4 µg/mL. Reductions in MIC of two dilutions or more were considered to exhibit “acquired resistance,” which either did or did not produce beta-lactamase. Isolates with borderline resistance were considered as a type of resistance that may be treated by modified (MOD) penicillin-binding proteins or unknown mechanisms. If the MBC or MIC rate was equal to or greater than 32, the isolates were defined as “tolerant” isolates (11).
4. Results
Of the 105 S. aureus isolated, 45 were obtained from female in-patients, while 60 isolates from male in-patients. Antibiotic susceptibility tests by the disk agar diffusion method utilizing a cefoxitin disk showed that 39 (37.1%) isolates were MRSA, while 66 (62.8%) isolates were observed to be MSSA. The most frequent source of these MRSA strains included wounds (56.4%) followed by blood (25.6%; see Table 1).
| Specimens | No. (%) of Isolates | No. (%) of MRSA Isolates |
|---|---|---|
| Wounds | ||
| Burn unit | 15 (14.2) | 12 (80) |
| Other wards | 35 (33.3) | 10 (28.5) |
| Synovial fluid | 5 (4.7) | 3 (60) |
| Urine | 9 (8.5) | 2 (22.2) |
| Peritoneal fluid | 6 (5.7) | 2 (33.3) |
| Blood | 27 (25.7) | 10 (37) |
| Abscesses | 8 (7.6) | 0 (0) |
| Total | 105 (100) | 39 (37.1) |
Distribution of MRSA in Various Clinical Specimens
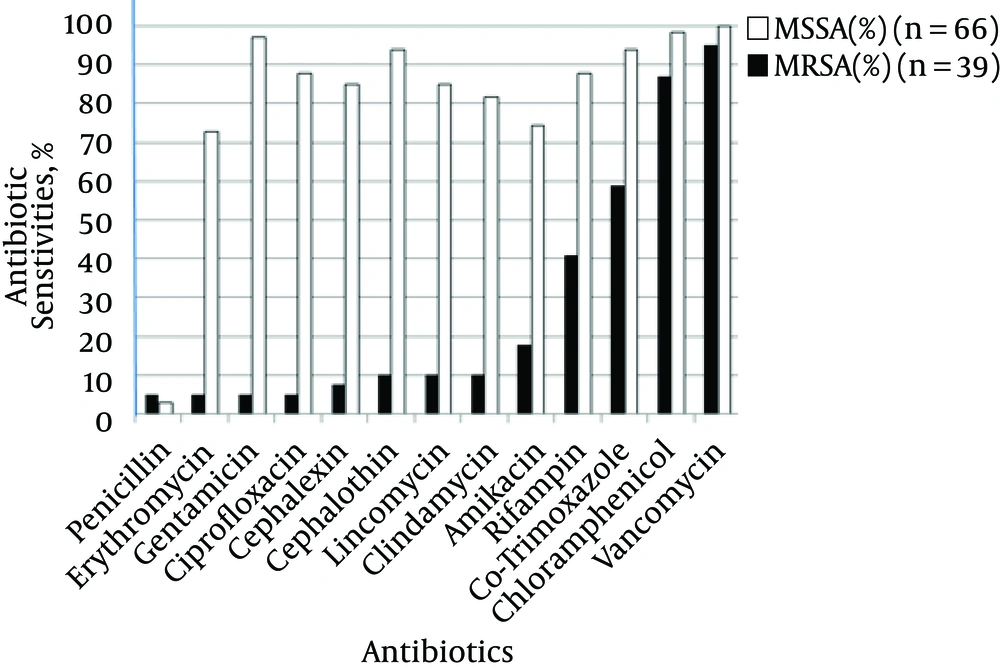
Antibiotic susceptibility tests revealed the high sensitivity of MRSA isolates to vancomycin (94.8 %), chloramphenicol (87.1%), and sulfamethoxazole (58.9%) as well as low susceptibility to penicillin, erythromycin, gentamicin, and ciprofloxacin (5.1% sensitivity for each stated antibiotic). All MSSA isolates were susceptible to vancomycin (100%), while sensitivity to chloramphenicol, gentamicin, co-trimoxazole, and cephalothin ranged from 93.9 to 98.4%. Among MSSA isolates, all except two isolates were found to be resistant to penicillin (Figure 1). Vancomycin susceptibility was checked by an E-test, and MICs of all resistant isolates, which ranged from 4 to 8 μg/mL, were considered as vancomycin-intermediate S. aureus (VISA). Of the 39 MRSA, 33 were also found to be clindamycin resistant 21 isolates showed no zones around clindamycin, and 12 (36.3%) isolates revealed D-test positivity for disk agar diffusion while among MSSA isolates, 10 (15.15%) isolates were found to be resistant.
To determine intrinsic resistance in MRSA, agar dilution and agar screen methods using oxacillin were used. Oxacillin MICs of all 66 MSSA isolates were observed to be less than 2 μg/mL and were confirmed as methicillin-sensitive isolates. None of the isolates presented oxacillin MICs between 2 - 8 μg/mL, so no isolate was considered to have borderline or acquired resistance. However, 39 MRSA isolates that revealed an MIC > 8 μg/mL were regarded as intrinsic resistant. No tolerant isolate (MBC/MIC > 32 μg/mL) was encountered. Of the total isolates studied, 41 isolates grew on oxacillin agar screening plates without any added NaCl, but 40 isolates showed the presence of bacterial growth on oxacillin agar screening plates with added NaCl.
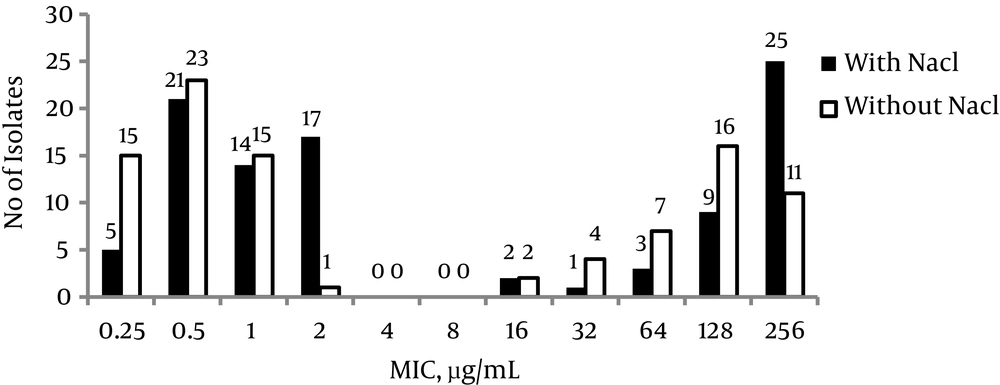
To assess the role of NaCl in the expression of resistance, the isolates were tested by the addition or omission of salt in the agar dilution test. In 58 isolates (55.2%), NaCl had no effect on the MIC, while 12 (24.4%) MRSA isolates and 34 (51.5 %) MSSA isolates showed an increase of one or two titer MICs in the presence of NaCl (Figure 2). When the effect of clavulanic acid was evaluated, it was found that MIC values decreased two- to four-fold in the presence of an inhibitor without NaCl, while with NaCl, the decrease was one- or two-fold.
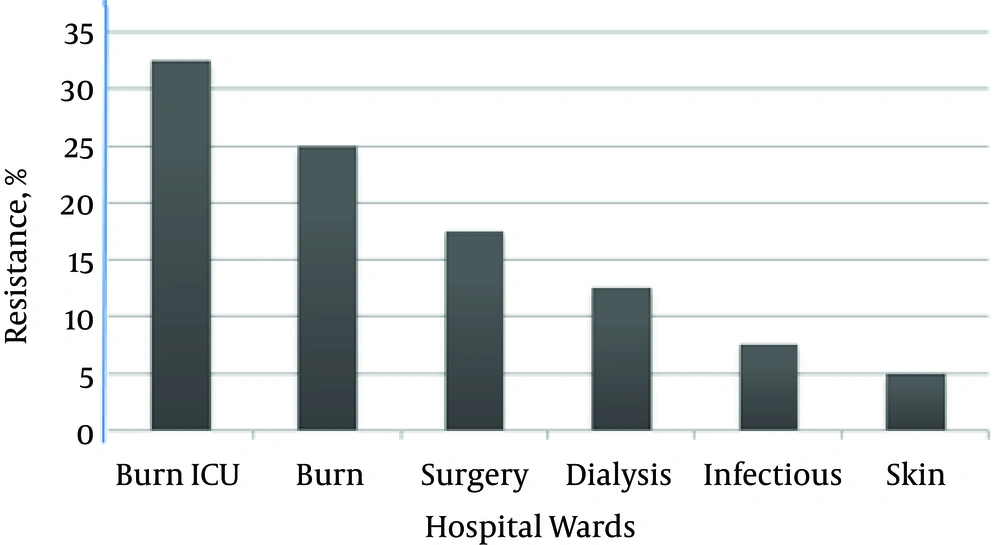
Regarding hospital wards, MRSA isolates were more prevalent in the ICU burn (32.5%) and burn (25%) wards in comparison to the skin (5%) ward. Figure 3 shows the distribution of intrinsic resistance in the isolates from different hospital wards. Multi-drug resistance (MDR) in MRSA was observed in 92.5% of the isolates, while only 20% of the MSSA isolates demonstrated MDR. MDR isolates showed varied resistance to 4 - 8 antibiotics simultaneously.
5. Discussion
S. aureus is an important pathogen conventionally isolated from various wards in the hospital setting. On the other hand, a high percentage of MRSA in healthcare centers, especially for patients who do not exhibit any symptoms or signs of severe disease, is also very dangerous (12). Antibiotic resistance is another factor considered for repeated colonization (13), and it is mandatory for every hospital or clinical setting to perform an accurate detection of MRSA. Knowledge of the prevalence of antibiotic resistance is a pre-requisite for infection control, and national guidelines for treatment are essential for the policy makers of public healthcare to conduct effective responses (14-16).
In the present study, among 105 S. aureus isolates, 38% were identified as MRSA and 62.8% were MSSA. The highest resistance recorded in the isolates was collected from the wound discharge and blood specimens of patients admitted to an ICU burn ward. Possible explanations include the widespread use of antibiotics (especially β-lactams), the immunocompromised status and/or prolonged hospital stay of patients, and the lack of control and screening of hospital personnel, as we discussed in an earlier publication (12). Among the S. aureus isolates, 38% were classified as intrinsic-resistant MRSA, indicating their emergence as important endemic pathogens in the hospitals selected for the study. Although a high rate of tolerant isolates has been reported in some studies (17), no tolerant isolate was encountered in this study, which is in agreement with other studies performed in Iran (18). No acquired resistance was found in in our investigation, which may be due to the enrollment of S. aureus isolates from in-patients only or to the increased resistance of these isolates in the hospital population.
In the present investigation, the results obtained by disk agar diffusion and agar screen plates were compatible except for a single isolate in which resistance was detected only by the agar screening method. An absolute correlation existed between the agar screen plate and the MIC determination as well. Thus, the agar screen plate can be recommended as the method of choice for routine laboratory identification of MRSA.
Regarding the use of NaCl for the accurate detection of MRSA, it is hypothesized that NaCl stimulates the production of PBP-2a, which in turn increases the sensitivity of the test (10). However, we did not find any effects for NaCl in 55.2% of isolates. Similarly, when studying the effects of NaCl on decreases in oxacillin MICs and inhibitor results, no remarkable influence was noticed in our study. Thus, it can be concluded from our results that routine laboratory tests can obtain accurate results by omitting NaCl.
As per the patterns of antibiotic sensitivity, all MSSA isolates were sensitive to vancomycin, while 98.4% and 96.9% were sensitive to chloramphenicol and gentamicin, respectively. In our study, neither MSSA nor MRSA isolates showed good promise for the use of penicillin as only 5% of MRSA and 3% of MSSA isolates were sensitive to this antibiotic.
MRSA isolates were the least susceptible (5.1%) to antibiotics such as gentamicin, erythromycin, and ciprofloxacin. Chloramphenicol, vancomycin, and other antibiotics used in this study were more effective against MRSA and MSSA isolates. But some MRSA isolates, the so-called VISA, had borderline resistance to vancomycin. It appears that assessing MRSA strain sensitivity to other antibiotics, such as linezolid, may be necessary in future studies.
In a systematic review conducted in Iran and another studies, the methicillin-resistance rate has been disclosed as between 42% - 47% (17-19). The observations of these studies are similar to our results. A study performed in Shiraz (2000) reported 33% of S. aureus as MRSA, and the sensitivity of these MRSA strains to vancomycin and rifampin were reported as 100%, while all isolates were found resistant to penicillin (20). Other studies from Turkey and Libya reported a somewhat higher frequency of MRSA (56% in Turkey and 59% in Libya). The percentage of isolates resistant to vancomycin in Libyan hospitals (7%) compared with hospitals in Turkey (2.2%) was also higher (20). The percentage of methicillin resistance in our study is lower than the aforementioned studies; although we did not find vancomycin-resistant isolates, we did find an overt creep in vancomycin. In terms of multi-drug resistance, our study is similar to both aforementioned studies. In 2010, Peng et al. (23) studied 115 isolates of S. aureus with both PCR and antibiotic sensitivity testing according to CLSI standards at a hospital in China; the researchers found that all isolates had a high resistance to ampicillin, oxacillin, gentamicin, erythromycin, and ciprofloxacin and a low resistance to doxycycline (6%). All isolates were sensitive to vancomycin (21), which is the drug of choice for treating MRSA infections (22).
The emergence of MRSA clinical isolates resistant to vancomycin has been reported (23). This resistance is important since it reveals the necessity for a review of diagnosis and treatment more than ever. In the present study, although vancomycin-resistant MRSA isolates were not detected, 6 VISA isolates were observed, which is alarming for the near future. The MRSA isolates in this study were sensitive to the most commonly used antibiotics against staphylococcal infections. Thus, methicillin resistance is considered a valid indicator to design a treatment plan and select the appropriate antibiotic to treat infections caused by S. aureus.
5.1. Conclusion
Our results revealed that the prevalence of MRSA strains is increasing in high-risk wards. The emergence of such antibiotic-resistant isolates is a growing concern. For the detection of intrinsic or acquired resistance, the addition of NaCl does not significantly affect the results. In addition to the use of cefoxitin, oxacillin agar can serve as a reliable test for methicillin resistance. Rapid identification of MRSA isolates and surveillance of antibiotic susceptibility patterns is essential.