1. Background
Sepsis is the main cause of mortality in neonates (1, 2). Neonatal deaths account for 40% of deaths in children under the age of five years worldwide (3), and neonatal mortality due to sepsis is increasing (4). Because neonatal sepsis entails multi-organ system involvement and widespread manifestations can overlap with many other disorders during multiple stages of life, over diagnosis and overtreatment in neonatal sepsis may occur. Although antibiotic usage is popular in NICUs, this can cause growing resistant strains of microorganisms (2, 5). The increased survival rate of neonates due to improved care of critically-ill infants especially preterm neonates and neonates with surgical problems has led to prolonged hospital stays and prolonged usage of broad-spectrum antimicrobial agents, which in turn has led to microbial resistance (6). In most cases of neonatal sepsis, selection of antibiotics in the NICU is not based on known microorganisms or antibiograms. In some patient cases that call for empiric antibiotic regimens, critically-ill neonates die or recover before the results of blood cultures can be obtained.
Some researchers have shown that changing microbial patterns and antibiotic sensitivities have been occurring in recent decades (7, 8). The risk factors for these changes are prolonged use of antibiotics, long-term hospital stays, surgery, and more invasive manipulations in neonates. Information about changes in and assessment of antimicrobial sensitivity patterns over time facilitates the selection of the most appropriate antibiotics. The NICU of the Mofid Children’s hospital is a training level 3 intensive care unit with medical and surgical cases. Most of the patients in our NICU receive broad-spectrum and prolonged antibiotics. We propose that the microbial patterns and antibiotic sensitivities in this population have changed over time.
2. Objectives
The objective of our study was to detect changes in microbial patterns and antibiotic susceptibilities reflected in blood cultures collected during two periods of time (1992 and 2015) in the Mofid Children’s hospital.
3. Methods
In this research, we conducted a retrospective comparative descriptive study between the years 1992 and 2015 (a 23-year interval) about microbial characteristics and antibiograms of blood cultures in neonates admitted in to the NICU of the Mofid Children’s hospital in Tehran, Iran. Our NICU has 18 beds and is covered by the Shahid Beheshty Medical University of Science. All of the patients are outborns, and many patients with surgical problems (except those requiring heart surgeries) are referred from Tehran and other distant cities. Inclusion criteria were as follows: all neonates with proven sepsis with positive blood cultures during an old study in 1992 and another in 2015. Data were extracted from the medical records of patients and laboratory software systems of the Mofid hospital belonging to neonates who were admitted to the NICU with positive blood culture, regardless of their surgical or medical problems. Blood cultures were performed in traditional bacterial media. However, we did not use Bactec media as a routine in the evaluation of neonatal sepsis. Antimicrobial susceptibility testing was done by antibiotic disc method. We did not use media appropriate for viral or anaerobic infections. Demographic data of patients such as gestational age, birth weight, sex, and underlying disease, type of microorganisms, and sensitivity and resistant rate of antibiotics were also analyzed. Statistical analysis was carried out by using SPSS version 21.
4. Results
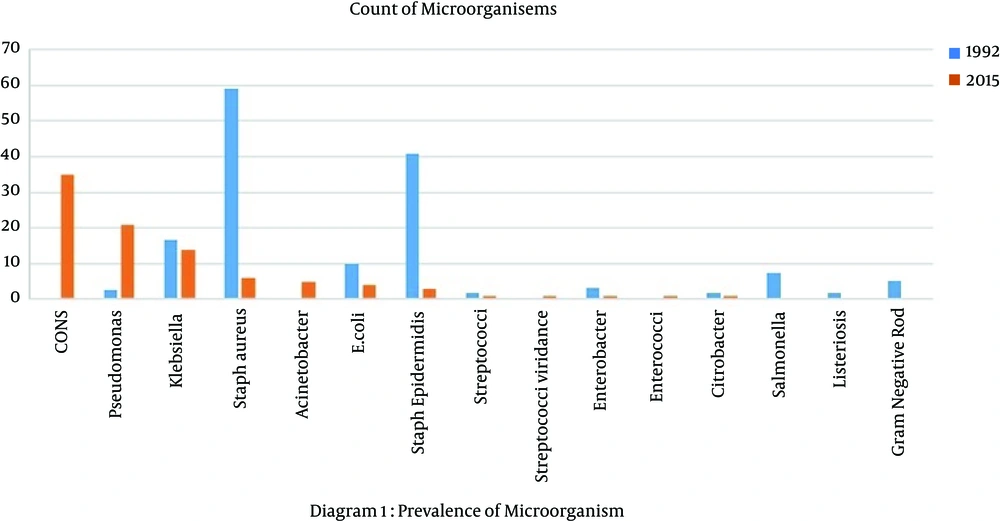
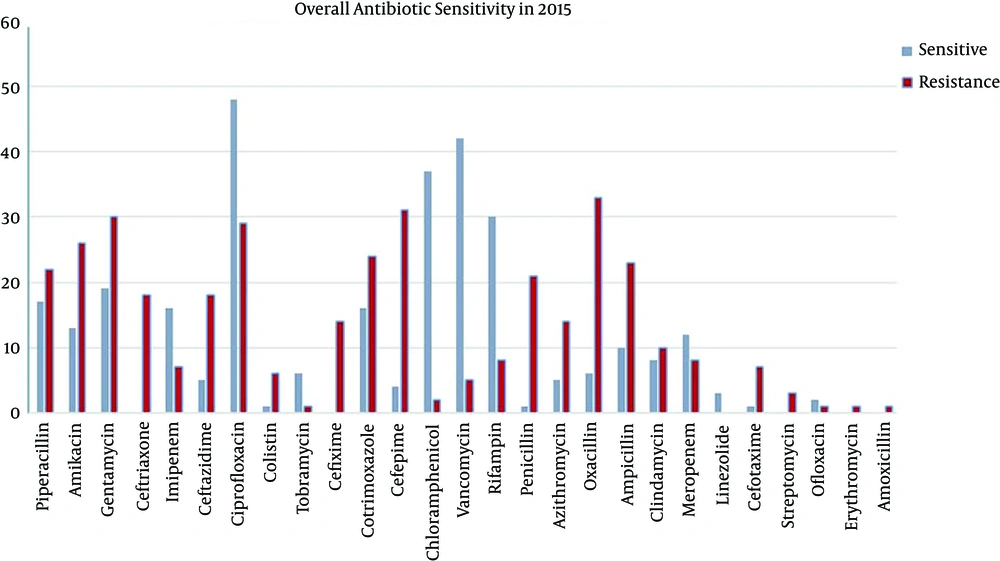
In this study, we compared the results of 100 positive blood cultures collected in 1992 with those of 103 samples collected in 2015. Overall, 57% of neonates were male and 43% were female; 56% of sepsis cases were late onset (after first week of life) and 44% were early onset (first week of life). Sixty-three percent of neonates had term gestation (more than 37 weeks of pregnancy) and 37% were preterm (less than 37 weeks of gestation). The microorganisms detected in the blood cultures during both years are showed in Figure 1, and the sensitivity patterns of commonly used antibiotics are illustrated in Figure 2. Because of the low rate of intermediate susceptibility in antibiograms, their results were ignored.
We found that the most common causes of positive blood cultures isolated in 1992 were Staphylococcus aureus (59%), Staphylococcus epidermidis (40.9%) and Klebsiella (16.6%). In 2015, coagulase negative Staphylococci (CONS) (33.98%), Pseudomonas aeruginosa (20.3%) and Klebsiella (13.5%) were the most common microorganisms (Table 1). The prevalences of Acinetobacter and Enterobacter sepsis in 2015 were 4.58% and 0.97%, respectively, whereas the prevalences in 1992 were zero. Salmonella was present in 7.5% of cases of 1992, but it was not present in any samples in 2015 (Table 1).
| Microorganism-Antibiogram | Staphylococcus aureus | Staphylococcus epidermidis | Klebsiella | E. coli | Gram Negative |
|---|---|---|---|---|---|
| Cephalotin | 45% | 100% | 35% | 35% | 0 |
| Penicillin/ampi | 0 | 0 | 0 | 0 | 0 |
| Cloxacilin | 50% | 0 | - | - | - |
| Oxacillin | 22% | 0 | - | - | - |
| Gentamycin | 0 | 12% | 8% (inter) | - | 25% |
| Tobramycin | 0 | 33% | 0 | 65% | 0 |
| Kanamycin | 0 | 0 | 25% | 50% | 25% |
| Amikacin | 100% | 75% | 100% | 75% | 100% |
| Erythromycin | 43% | 75% | - | ||
| Bactrim | 50% | 0 | 45% | 45% | 25% |
| Chloramphenicol | - | 100% | 30% | 20% | - |
| Nitrofurantoine | 45% | 75% | 65% | ||
| Nalidixic acid | 85% | 50% | - |
Because of the restrictive usage of antibiotics in the past in comparison to recent years, we did not have the results of antibiograms of recently used antibiotics such as meropenem, ciprofloxacin, colistin, piperacillin in 1992. Based on the available results, we categorized antibiotic susceptibility as follows:
4.1. Aminoglycoside
In comparison to available antibiotic susceptibility, despite the high consumption of aminoglycoside in NICU and neonatal wards in recent antibiograms in 2015, resistance for amikacin was higher than sensitivity: 25.2% vs. 12.6% (Figure 2), and these results are very different from those obtained in 1992, at which time 75-100% sensitivity was observed (Table 1). The resistance rate and sensitivity rate to gentamycin in 2015 were 29.1% and 18.4%, respectively (Figure 2) in comparison to 1992, at which time 8–25% of microorganisms were sensitive to it (Table 1).
4.2. Cephalosporins
Among cephalosporin-treated groups in 2015, sensitivity and resistance rates to ceftazidim were 4.9% and 17.5%, respectively; to sefepim were 3.9% and 30.1%, respectively; and to sefotaxim were 1% and 6.8%, respectively (Figure 2). In 1992 antibiograms for cephalotin revealed 45% and 100% sensitivity against Staphylococcus aureus and Staphylococcus epidermidis, respectively and 35% against E. coli and klebsiella (Table 1).
4.3. Anti-Staphylococcal Antibiotics
In 1992, cloxacillin and oxacillins were assessed, and 22% and 50% of microorganisms were found to be sensitive, respectively. No results were obtained about vancomycin (Table 1). In 2015, the rates of sensitivity and resistance to vancomycin were 42% and 4.9%, respectively and to oxacillins were 5.8% and 32%, respectively (Figure 2).
4.4. Broad-Spectrum Antibiotics
Sensitivity and resistance rates were as follows: meropenem, 11.7% vs. 7.8%; imipenem, 15.5% vs. 6.8%, ciprofloxacilin, 46.6% vs. 28.2%; colistin, 1% vs. 5.8%, respectively (Figure 2). One of the most common antibiotics used in NICUs and neonatal wards is ampicillin, which had a 22.3% resistance rate and a 9.7% sensitivity rate, according to blood cultures.
Table 2 shows antibiotic sensitivities based on microorganisms cultured in 2015. As shown in the table, the broad-spectrum antibiotic piperacillin effectiveness rates were 42.9%, 35.7%, and 25% against Pseudomonas aeroginousa, Klebsiella, and E. coli, respectively without any effectiveness against Acinetobacter. Ciprofloxacin had the highest effectiveness against E. coli, Klebsiella, and Staphylococcus epidermidis (75%, 71.45%, and 66.4%, respectively). Evaluation of microbial sensitivity to vancomycin revealed the following sensitivity rates: 100% for Streptocci, 83.3% for Staphylococcus aureus, and 71.4% for coagulase negative Staphylococci (Table 2).
| Microorganism | Acinetobacter | Coag Neg Staph | E. coli | Enterococci | Klebsiella | Pseudomonas | S. aureus | S. epidermidis | Strep | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | S | R | |
| Piperacillin | - | 100 | - | - | 25 | 50 | - | - | 35.7 | 42.9 | 42.9 | 38.1 | - | - | - | - | - | - |
| Amikacin | - | 60 | - | - | 75 | 25 | - | - | 14.3 | 64.3 | 28.6 | 52.4 | - | - | - | - | - | 25 |
| Gentamycin | 20 | 80 | - | - | 50 | 50 | 20 | 60 | 28.6 | 71.4 | 42.9 | 33.3 | - | - | - | - | - | 50 |
| Ceftriaxone | - | - | - | - | - | 50 | - | - | - | 42.9 | - | 42.9 | - | - | - | - | - | - |
| Imipenem | - | 40 | - | - | 50 | - | - | - | 57.1 | 7.1 | 23.8 | 19 | - | - | - | - | - | - |
| Ceftazidime | - | 40 | - | - | - | 75 | - | - | 14.3 | 35.7 | 4.8 | 38.1 | - | - | - | - | - | - |
| Ciprofloxacin | - | 100 | 31.4 | 48.6 | 75 | - | 40 | 20 | 71.4 | 7.1 | 57.1 | 19 | 50 | 16.7 | 66.7 | - | 50 | - |
| Colistin | - | - | - | - | - | - | - | - | - | 14.3 | 4.8 | 19 | - | - | - | - | - | - |
| Tobramycin | - | 20 | - | - | - | - | - | - | 7.1 | - | 19 | - | - | - | - | - | - | - |
| Cefixime | - | - | - | - | - | 75 | - | - | - | 35.7 | - | 28.6 | - | - | - | - | - | - |
| Cotrimoxazole | - | - | 4.3 | 42.9 | - | - | 20 | 20 | 21.4 | 7.1 | 14.3 | 4.8 | 33.3 | 16.7 | 66.7 | 33.3 | - | 75 |
| Cefepime | - | 80 | - | - | 25 | 50 | - | - | 14.3 | 64.3 | - | 71.4 | - | - | - | - | - | - |
| Vancomycin | - | - | 71.4 | 8.6 | - | - | 60 | 40 | - | - | - | - | 83.3 | - | 66.7 | - | 100 | - |
| Rifampin | - | - | 54.3 | 20 | - | - | 40 | 20 | - | - | - | - | 83.3 | - | - | - | 25 | - |
| Penicillin | - | - | - | 45.7 | - | - | 20 | - | - | - | - | - | - | 50 | - | 33.3 | - | - |
| Azithromycin | - | - | 8.6 | 34.3 | - | - | - | - | - | - | - | - | - | 33.3 | 33.3 | - | - | - |
| Oxacillin | - | - | 8.6 | 85.7 | - | - | - | - | - | - | - | - | 33.3 | 16.7 | 33.3 | 33.3 | - | - |
| Ampicillin | - | - | 5.7 | 34.3 | - | - | 40 | 20 | 7.1 | 21.4 | - | - | - | 66.7 | 33.3 | 33.3 | 50 | 25 |
| Clindamycin | - | - | 5.7 | 22.9 | - | - | 20 | - | - | - | - | - | 33.3 | 16.7 | 33.3 | - | 25 | - |
| Meropenem | - | 80 | - | - | 25 | - | - | - | 50 | - | 19 | 19 | - | - | - | - | - | - |
| Linezolide | - | - | 2.9 | - | - | - | 20 | - | - | - | - | - | - | - | 33.3 | - | - | - |
| Cefotaxime | - | - | - | - | 25 | 25 | - | - | - | 7.1 | - | 19 | - | - | - | - | - | - |
| Streptomycin | - | - | - | - | - | - | - | 40 | - | - | - | - | - | - | - | - | - | - |
Abbreviations: S, Sensitive; R, Resistant.
One-hundred percent of Acinetobacters and 42.9% of Klebsiella were resistant to piperacillin (Table 2).
Due to the increasing use of amikacin, in 1992, 100% of Klebsiella and gram negative bacilli, 75% of E. coli, and 75 - 100% of Staphylococcus were sensitive to amikacin (Table 1), whereas in 2015, approximately 60% of Acinetobacter and Klebsiella and 52% of pseudomonas were resistant to amikacin (Table 2).
Ascinetobacter results in devastating nosocomial infection with a high level of resistance. Our study showed that in 100% cases of Ascinetobacter infection, antibiograms revealed resistance to piperacillin. They also revealed 60% resistance to amikacin, 80% to gentamycin, 40% to imipenem, 80% to meropenem, 80% to ceftazidim, 100% to ciprofloxacillin, and 80% to cefepime. Gentamycin was the only suitable drug for treatment of this microorganism (Table 2).
Staphylococci coagulase negative was resistant and sensitive to vancomycin at rates of 8.6% and 74.1%, respectively and resistant and sensitive to clindamycin at rates of 22.9 and 5.7%, respectively (Table 2).
Pseudomonas aeruginosa was resistant to piperacillin and ceftazidime at a rate of 38.1%, to amikacin at a rate of 52.4%, and to cefepime at a rate of 71.4% (Table 2).
Klebsiella was resistant to gentamycin at a rate of 71.4%, to amikacin and cefepim at a rate of 64.3%, and to imipenem at a rate of 7.1% (Table 2).
5. Discussion
In the current comparative study, which took place in the NICU of the Mofid hospital, we evaluated the pattern of microbiology and antibiogram sensitivity between two years, with a 23-year interval between them. Our study showed that the rate of resistance to aminoglycosides and cephalosporins increased. Because the first antibiotic regimens in neonatal early and late onset sepsis require these two commonly used antibiotics, such an increase in microbial resistance is predictable. Similar to our study, the research of Marzban in 2010 (7) showed that when microbial patterns in 1990 - 1992 and 2004 - 2007 were compared, the causes of neonatal sepsis had changed, meaning that during the first period of study in 1992, the most commonly detected bacteria were gram negative bacteria (66%) and Staphylococcus aureus (34%). During the second period in 2014, 56.6% of the bacteria were gram negative, and bacterial resistance had increased.
In our study, staphylococci were the most common microorganisms during both years; however, there were no cases of Ascinetobacter or Enterococci in blood cultures from 1992, but 4.5% and 0.97% of the cultures in 2015 were positive for Ascinetobacter and Enterococci, respectively. In addition, Pseudomonas aeroginousa was more prevalent in 2015 than 1992 (Figure 1).
The incidence of listeria monocytogen infection in neonates is 4 - 6%, and coverage of Listeria is recommended for treatment of neonatal sepsis; however, in our study, Listeria monocytogen was found in 1.72% of neonatal sepsis cases in 1992, but no cases were found in 2015. In some countries, such as the United States, there was a decrease in the incidence of this pathogen between 2003 and 2007; however, in in England, it increased (9). A relatively low incidence of listeria infection in the NICU of Mofid hospital may be due to higher admission rates of neonates with late onset sepsis and surgery problems. In the nursery and medical NICUs, the prevalence of listeria infection is probably greater than in our study.
Salmonella infection is rare in neonatal periods, but there are some case reports of it, such as those of Sharan and Kumar (10), who in 2013 reported two cases of Salmonella infection. In our study, we found that 7.23% of blood cultures in 1992 were positive for Salmonella, but no cases of Salmonella were found in 2015. This may be due to increasing levels of hygiene in families and more breast feeding of neonates.
Staphylococcal species most notably S. epidermidis and S. aureus cause approximately 60 - 70% of infections (11). The research of Hamer et al. (12) showed that Staphylococcus aureus was the most common cause of neonatal sepsis in 2015 in six countries: Bangladesh, Bolivia, Ghana, India, Pakistan, and South Africa. Our study showed that the Staphylococcal species was most common; in 1992, the most prevalent was Staphylococcus aureus, and in 2015, it was CONS.
Considering that surgery is a risk factor for staphylococcal infections and the Mofid hospital is a tertiary referral center for surgical problems, this result is acceptable. In this respect, it is important that appropriate antibiotics be selected to cover this microorganism. In 1992, antibiograms revealed that staphylococcal infection had sensitivity to oxacillin at a rate of 22 - 50%; however, in 2015, just 5.8% had sensitivity, and the rate of resistance increased to 22 - 50% in 2015. This indicates that the use of this antibiotic in critically-ill neonates with suspected methicillin resistant staphylococcal infection is not justified.
The results for vancomycin susceptibility are as follows: 40.8% sensitivity vs. 4.9% resistance; this is promising. The restrictive use of this drug in neonates explains this promising data. However, in a study by Lawrence et al. in 2006 (13), which compared of vancomycin and cloxacillin in late onset sepsis, cloxacillin was found to be as effective as vancomycin, and the researchers recommended restricting vancomycin for confirmed cases of CONS sepsis resistant to oxacillin. This significantly reduced vancomycin use in the NICU; however, this study was older than our research, and more resistant forms of microorganisms have since developed.
In a study conducted by Hsiu-Mei Wei in 2015 (14), multidrug resistant Acinetobacter in the NICU were evaluated, revealing the following: Acinetobacter was resistant to amikacin, ceftazidime, ciprofloxacin, cefpirome, cefepime, gentamicin, imipenem, levofloxacin, meropenem, ampicillin-sulbactam, trimethoprim/sulfamethoxazole, and piperacillin/tazobactam. However, Acinetobacter was sensitive to colistin and tigecycline. These results are similar to those of our study, in which sensitivity rates to antibiotics were very low and the resistance rates were high (100% for piperacillin and ciprofloxacillin, 80% for gentamycin and cefepim, 60% for amikacin).
In another study about Acinetobacter sepsis in newborns by Asit Mishra (15) in 1998, which was conducted in India, 251 positive blood cultures were analyzed, with the following results: 31.5% prevalence of Acinetobacter, 26.3% prevalence of E. coli, 10.7% prevalence of Klebicella, 7.2% prevalence of pseudomonas, 4% prevalence of CONS, and 16.3% prevalence of staph coagulase positive. These results were in contrast to the results of our study in 1992, which revealed a 59% prevalence of Staphylococcus aureus and a 40.9% prevalence of staph epidermis, with no cases of Acinetobacter.
We did not find any fungal infection cases in our study. This may be because of the low incidence of fungal infection in our NICU and the low sample size of the research.
We did not use viral or anaerobic cultures for evaluation of neonatal sepsis, and for this reason, we do not have any information about viral or anaerobic infection in our NICU.
Other limitations of our study include small sample size and the fact that not all antibiotic disks in the antibiogram plates were used in all positive blood cultures, and this can limit the accuracy of antibiotic susceptibility pattern analysis.
5.1. Conclusion
In this research, we found that the pattern of microbial and antibiotic sensitivity has changed, and overall antibiotic resistance is increasing. This is an indication that healthcare providers should use broad-spectrum antibiotics with caution; otherwise, perhaps a day will come when there are no antibiotics for resistant bacteria.