1. Background
Brain Abscess (BA) is an uncommon intracranial suppurative infectious disease, especially when occurring in immune-competent children belonging to the age group of four to seven years (1). It might cause long-term neurological deficits or even death. The diagnosis can be insidious with non-specific clinical symptoms, which depend on many factors, such as age, abscess location, and size (2). Commonly-isolated causative microorganisms are various, with predominance of anaerobic microorganisms. However, the microbiological spectrum has changed with increasing number of immune-compromised patients developing BA (3). In children, the optimal management of BA is still controversial. Obviously, the main treatment of pyogenic BA is prolonged antimicrobial medication, yet the decision for surgical excision or aspiration in combination versus conservative medical treatment alone is debated depending on healthcare providers (3). Surgical treatment should be considered based on the location, size, the clinical condition, and the chance of achieving successful decompression. The management of BA should take into consideration the context of the developing country’s health systems to reduce heavy economic burden, although large variations in data occur across studies (2, 3). More research concerning particularities of BA could therefore be relevant.
To the best of the author’s knowledge, little is known about the situation in North Africa.
2. Objectives
The objective of this study was to characterize the features of pyogenic BA in children diagnosed and treated in Tunisian health center and to determine the outcomes and prognostic factors.
3. Methods
This multi-center retrospective study included all children with BA treated in four different Tunisian pediatric departments of the central region of the country (Sahloul University hospital, Kairouan University Hospital, Farhat Hached University Hospital, and Fattouma Bourguiba University Hospital) from January 1995 to December 2014. All patients were referred to the same neuro-surgical department in Sahloul Hospital. The research included all patients with typical pyogenic BA on contrast Computed Tomography (CT) or Magnetic Resonance Imaging (MRI). This study also included patients with evidence of bacterial BA uncovered by neurosurgery or appropriate microbiological specimen. The BA content aspirated during surgery was transported immediately to the microbiology laboratory for processing in aerobic an anaerobic culture conditions (1). Patients with negative microbial findings were also part of the study. Mycobacterial, parasitic or fungal abscess cases and those with subdural empyema were excluded. The patient charts were reviewed including demographic characteristics, predisposing factors, presenting symptoms, underlying medical conditions, imaging data, isolated microorganisms, treatment, and outcome. A statistical analysis of prognostic factors was performed including outcome, death, and other factors (clinical, laboratory and radiology findings, and treatment modalities). Statistical analyses were performed using SPSS 10. Continuous variables are presented as mean ± standard deviation and categorical variables as percentages. Correlation of the risk factors with clinical findings was obtained by using the Pearson Chi-square test and Fisher's exact test, depending on the type of variable. A P value of less than 0.05 was considered statistically significant.
4. Results
Forty-one children with records available for analysis were diagnosed with BA between January 1995 and December 2014. The median age was 2.9 years (range: 4 days to 16 years) and 75% were male. The patient population included nine neonates (21%). Forty percent were under the age of two years.
Table 1 provides major presenting symptoms and signs; more than one symptom could be found in one patient at the same time. Fever was the most common symptom on initial presentation, noted in 58.5% of children. Headache, nausea and/or vomiting were the second most common presenting symptoms, noted in 41.4%. Seizure was also a common initial presentation, noted in 14 children (34.1%).
| Signs and Symptoms | N° | % |
|---|---|---|
| Fever | 24 | 58.5 |
| Headache | 17 | 41.4 |
| Nausea-vomiting | 17 | 41.4 |
| Seizures | 14 | 34.1 |
| Decreased level of consciousness | 7 | 17 |
| Nuchal rigidity | 9 | 21.9 |
| Brudzinski sign | 6 | 14.6 |
| Kernig sign | 6 | 14.6 |
| hypotonia | 3 | 7.3 |
| Focal neurological involvement | ||
| Hemiplegia | 3 | 7.3 |
| Hemiparesis | 1 | 2.4 |
| Paralysis of the upper limb | 2 | 4.8 |
| Cranial nerve palsy (facial, ocular, 6th cranial nerve palsies) | 5 | 12.1 |
| Ataxia | 1 | 2.4 |
| Diplopia | 1 | 2.4 |
Clinical Signs and Symptoms
Predisposing factors were identified in 34 cases (82.9%). Twenty-six abscesses were secondary to infections involving the brain and adjacent anatomical sites, including meningitis confirmed by lumbar puncture in 18 cases (43.9%), sinusitis in four cases (12.1%), Otitis Media in one case (2.4%), tooth abscess in two cases (4.8%) and orbital cellulitis in one case (2.4%). Three cases (7.3%) occurred after head trauma. Occipital dermal sinus was identified in one case. Four BA (9.7%) were developed in children with Cyanotic Congenital Heart Disease (CCHD) complicated in two cases with endocarditis and one (2.4%) with hip arthritis. No predisposing factor was found in seven cases (17.1%). There were no cases related to immunodeficiency.
Brain CT scan was performed in all patients whereas MRI was done only in 20 cases (48.7%). BA was diagnosed by imaging in 39 cases (95%). The other two patients were referred to surgery with the diagnosis of intra-cerebral tumor.
A single BA was detected in 33 cases (80.4%). Frontal and parietal lobes were most commonly involved (Table 2). Three cases were presented in the temporal lobe, two in Parieto-occipital lobe, and three abscesses were located in the cerebellum (Figure 1). Eight cases had multiple BA (Figure 2).
| N° | % | |
|---|---|---|
| Supratentorial | 38 | 92.7 |
| Frontal | 14 | 34.1 |
| Parietal | 7 | 17.1 |
| Temporal | 3 | 7.3 |
| Occipital | 1 | 2.4 |
| Fronto-parietal | 2 | 4.8 |
| Occipito-parietal | 2 | 4.8 |
| Occipito-temporal | 1 | 2.4 |
| Infratentorial (cerebellum) | 3 | 7.3 |
| Multiple | 8 | 19.5 |
Abscesses Localization
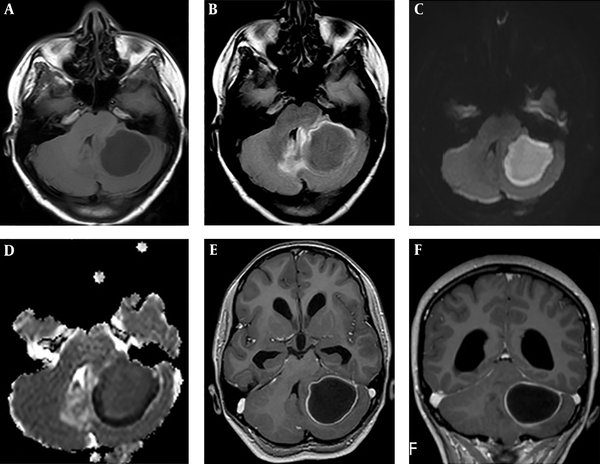
Cerebral MRI in a 10-year-old girl with brain abscess. A, Axial T1-weighted image before administration of contrast material shows well-defined lesion in the left cerebellum with central low intensity and isointense wall; B, Axial fluid-attenuated inversion recovery (FLAIR) weighted image shows central high intensity with peripheral high intensity (vasogenic oedema); C, Axial diffusio-weighted image shows marked hyperintensity in the abscess; D, ADC map reveals slight hypointensity, representing restricted diffusion in the corresponding region; E and F, Axial and coronal contrast-enhanced T1-weighted MR images shows a regular thin-walled ring-enhanced lesion.
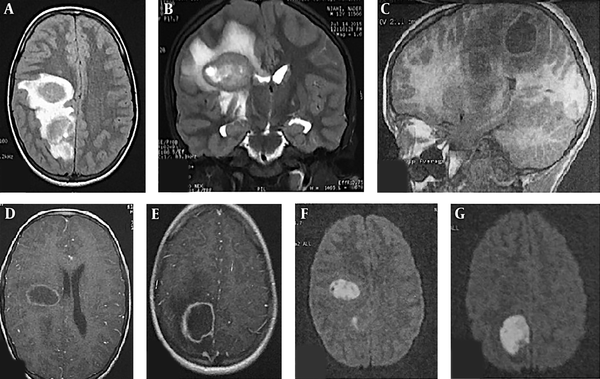
Cerebral MRI on a 12-year-old boy with two brain abscesses. A, Axial fluid-attenuated inversion recovery (FLAIR) weighted image shows two nodular frontal and parietal lesions with central high intensity and peripheral high intensity representing vasogenic oedema; B, Coronal T2-weighted image shows central hyperintensity with low intensity wall and peripheral vasogenic oedema; C, Sagittal T1-weighted image before administration of contrast material shows well-defined lesions with central low intensity and hyperintense wall; D and E, Axial contrast-enhanced T1-weighted MR images shows a regular thin-walled ring-enhanced lesions; F and G, Axial diffusion-weighted images shows marked hyperintensity in the abscesses.
Brain CT scan or MRI findings were corroborated by surgical intervention in only 26 patients (63%). Surgical intervention was performed immediately at diagnosis of abscesses with more than 20 mm of diameter in 19 patients and after a few days of anti-biotherapy in seven patients. In 18 of the 26 cases (69%), stereotactically guided aspiration was practiced and craniotomy excision for the others. Intravenous antimicrobial therapy alone was performed in 15 patients (36.6%) with multiple BA (six patients), deeply localized abscess (one case), and small size BA (eight cases). The antibiotic was parenteral in all cases.
The combination of cefotaxime, metronidazole, and fosfomycin was the most commonly used treatment (34.1%).
The treatment was reevaluated according to the results of Gram smear and abscess aspirate cultures. Imipenem was added in three cases and ofloxacin in two cases. Parenteral treatment was prolonged for six weeks in 15 cases (36.5%). Four patients (9.7%) were treated for four weeks, according to CT data.
Microorganisms were identified in 22 cases in different localizations (53.7%).
Pus cultures were performed in 26 patients (64.4%). Positive bacterial cultures were found in 14 (53.7%) of the pus samples: Staphylococcus (six cases), Streptococcus (three cases), and gram negative (four cases). Poly-microbial pathogens were isolated from one patient (Streptococcus milleri and Eikenella corrodens) (Table 3). Twenty-seven patients (65.8%) presenting initial signs pointing to meningitis, underwent lumbar puncture, with seven pathogens isolated from the cerebrospinal fluid culture (Table 4). The same microorganism was isolated in pus culture of two cases.
| Type of Microorganisms | N° of Cases |
|---|---|
| Gram-positive cocci | |
| 1. Streptococcus | |
| a) S. viridans | 2 |
| b) S. milleri | 3 |
| c) S. pneumoniae | 1 |
| 2. Staphylococcus | |
| a) S. epidermidis | 2 |
| b) S. aureus | 1 |
| Gram-negative bacilli | |
| 1- Proteus mirabilis | 1 |
| 2- Hoemophilus aphrophilus | 1 |
| 3- Citrobacter diversus | 1 |
| 4- E. coli | 1 |
| 5- Eikenella corrodens | 1 |
Pathogens Isolated in Abscess Pus Culture
| Type of Microorganisms | N° of Cases |
|---|---|
| Gram-positive cocci | |
| Streptococcus milleri | 1 |
| Gram-negative cocci | |
| Meningococcus | 1 |
| Gram-negative bacilli | |
| 1- Proteus mirabilis | 1 |
| 2- Hoemophilus b | 1 |
| 3- Citrobacter diversus | 1 |
| 4- E. coli | 2 |
Pathogens Isolated in Cerebrospinal Fluid
Blood cultures were performed in 34 patients (82.9%), yet they were positive in only two cases (5.8%), one with endocarditis and the other with hip infection.
The mortality rate was 24.4%. Death was due to nosocomial bacteremia in 40%. Clinical follow-up ranged from 24 to 70 months. The patient outcomes were favorable (clinical and radiological improvement without any neurological sequelae or moderate one with complete autonomy depending on age) in 17 patients (41.5 %) and ongoing neurological sequelae were found in 14 patients (34.1%).
The statistical analysis of prognostic factors showed no significant association between poor outcome, death, and other clinical factors including focal neurological deficits, fever, laboratory and radiology findings, and treatment modalities (Table 5). Only age of less than two years was identified as a statistically significant prognostic factor (P = 0.024).
| Prognostic Factors | Number of Cases | P | |
|---|---|---|---|
| Favorable Outcome | Unfavorable Outcome | ||
| Age, y | 0.024 | ||
| ≤ 2 | 4 | 11 | |
| > 2 | 17 | 9 | |
| Sex | 0.718 | ||
| Male | 15 | 16 | |
| Female | 6 | 4 | |
| Diagnostic delay | 1 | ||
| ≤ 7 j | 14 | 14 | |
| > 7 j | 7 | 6 | |
| Consciousness | 0.662 | ||
| Normal | 4 | 2 | |
| Altered | 17 | 18 | |
| Number of abscesses | 0.453 | ||
| Multiple | 3 | 5 | |
| Unique | 18 | 15 | |
| Etiology | 1 | ||
| Known | 17 | 17 | |
| Inknown | 4 | 3 | |
| Bacteriology | 1 | ||
| Positive culture | 11 | 11 | |
| Negative culture | 10 | 9 | |
| Treatment | 1 | ||
| Antibiotherapy | 8 | 8 | |
| Antibiotheray and surgery | 13 | 12 | |
| Surgical treatment | 0.41 | ||
| Aspiration | 10 | 7 | |
| Excision | 3 | 5 | |
The Main Statistical Analysis of Prognostic Factors
5. Discussion
The presentation of BA in infants is not specific (4, 5). However, headache, fever and vomiting, each occur in 60% to 70% of the patients (6, 7). The clinical manifestations in the current patients were compatible with the results of a number of other analogous studies (4, 7-9).
The most common underlying conditions in developed countries are sub-acute and chronic otitis media, mastoiditis, and congenital heart disease. However, their role has declined with the introduction of pneumococcal vaccination and administration of antimicrobial therapy for ear infections (10). In Tunisia, pneumococcal vaccination is still not introduced in infant immunization programs. A predisposing factor was identified in 82.9% of patients. In contrast to the most commonly described predisposing factors, meningitis and sinusitis were the most common predisposing factors followed by CCHD, in discordance with most published reports (1, 4). This cannot be explained by the high rate of CCHD in Tunisia. In fact, birth incidence of CCHD in the Tunisian population is in line with the general estimates in the world. However, a high rate of mortality (23%) was reported because of the lack of medical and surgical care (11). Differences between studies may also be related to different patients recruitment with 35% of patients aged less than two years. In Tunisia, meningitis affects children less than two years old with relatively high frequency (12).
An occipital dermal sinus and a congenital defect arising from neural tube closure failure, was identified in one case. A few cases of the association with BA have been reported (13, 14). This underlines the importance of early detection of congenital dermal abnormalities along craniospinal axis by routine examination of newborns (14).
The current patients had more frontal and parietal abscesses. This finding was similar to other studies (1, 4, 15). The specificity of the current findings was a high rate of multiple BA (19.5%).
Negative cultures totaled 46.3%, with sterile pus found in 46%. It represents a high rate of sterile cultures that have been described in others studies (4, 16, 17). There are different possible reasons for the high culture negative rate in the current series. First, abusive antibiotic use in Tunisia is common. Second, the intracranial pus samples may not have been transported to the microbiology laboratory quickly enough to be successfully analyzed. Third, before abscess fluid was sampled, 37% of the patients had undergone antimicrobial administration.
Nine (22%) of the patients had pathogens including Streptococcus and Staphylococcus in the cultures. Five (12.2%) of the patients had gram negative bacilli, in concordance with the literature (1, 3). In children, the causative pathogens were aerobic and anaerobic streptococci (60% to 70% of cases), gram-negative anaerobic bacilli (20% to 40%) followed by Enterobacteriaceae (20% to 30%) and Staphylococcus aureus (10% to 15%) (3).
The treatment of BA requires a combination of antimicrobials and surgical interventions (7). Antibiotics are always necessary to manage BA, either alone or associated with surgical intervention (18).
In the current study, 15 patients (36.5%) were treated with antimicrobial therapy alone. The current ratio represents a relatively high rate of isolated medical therapy. This may be explained by the difficulty in surgical drainage for multiple, small, and deeply localized BAs in Tunisian Surgical Departments. Recent studies (4, 15) considered that surgical treatment should be attempted in all BA cases, except during the stage of cerebritis. This not only allows achievement of a reduction of the mass effect, yet also identifies infecting pathogens. Precocious culture of abscess material provided during surgery is the best opportunity to make a microbiological diagnosis (15). However, surgery can be avoided by use of a minimally invasive radiologic method. Aspiration of the pus can be achieved through a burr hole under CT guided stereotaxy or real-time ultrasound (19). Those modalities are not yet used in Tunisia.
The treatment duration was usually guided by regression of abscess as verified by CT or MRI (19). In the current series, all patients had neuro-imaging follow-up. CT scan is a more available technique in Tunisia in emergency conditions and has been proved as a valuable asset in the diagnosis of BA. This imaging modality allows localization of the abscess and demonstration of any associated edema or mass effect. However, improvement in CT scans is generally observed within an average of 2.5 weeks and complete resolution of BA occurs in an average of 3.5 months. Radiographic abnormalities may persist for months after successful therapy, making useless the CT scan control before three weeks of intravenous antibiotic therapy (9). Moreover, Park et al. demonstrated that MRI plus FDG-PET improved the accuracy of assessing therapeutic responses to antibiotics treatment of brain abscess and aided in optimizing therapy (20).
There are a few recent recommendations about the duration of antibiotic therapy in the pediatric population. The standard duration of antibiotic therapy is four to six weeks (3). Indeed, Helweg-Larsen et al. (21) reported no cases of recurrence in patients with postsurgical antibiotic treatment limited to less than six weeks. However, Sharma et al. (22) report an association of short duration (< 3 weeks) or choice of oral antibiotic therapy with recurrence of BA among eight patients. Recently, Chengyu Xia et al. (18) showed that short-course intravenous antimicrobial administration in the adult population can be considered as a standard therapy for bacterial BA in the surgically treated group for 10 to 14 days. According to this data, the long duration of intravenous antibiotic therapy could be shortened. Neuro-imaging follow-up and the resolution of BA is a mandatory simultaneous condition. The emergence of imaging technologies, improved microbiological techniques, and prompt antibiotic and surgical management reduced mortality rates to 5% to 10% (23). In the current series, there was a high rate of mortality (24%). However, mortality directly due to BA in the current series was 14.6%, similar to other studies (1, 4).
The high rate mortality is attributed to the percentage of nosocomial bacteremia. The current findings highlight the need to intensify the fight against nosocomial infections in Pediatric Intensive Care Units, especially in developing country. The duration of hospitalization exposes to a high risk of nosocomial infections (24). However, the choice between prolonged antimicrobial administration therapy with possible additive side effects and shorter duration of antibiotic therapy in children with possible higher rate of recurrence remains controversial.
5.1. Conclusion
Pediatric BA treatment is still a public health challenge in developing countries. Predisposing factors for BA in children are different depending on the health system development level. The mortality rate is still high in Tunisia with a high rate of nosocomial infections compared to a recent multicenter study (25). The therapeutic strategy based on intravenous antibiotic therapy associated with the surgical intervention in some cases should be adapted to Tunisian context. The current study highlights the need for standardized national guidelines or adequate recommendations on type and especially intravenous duration of antibiotic treatment.
The most significant determinant of poor outcome was age of less than two years. However, the findings of results in this case series is limited by the selection biases inherent to a retrospective study and the number of patients.