1. Background
Leishmania typically is vector-borne zoonosis, with rodents and canids as common resevior hosts and humans as incidental hosts. Current challenges include the emergence of leishmaniasis in the new geographic areas and host populations (especially in HIV-positive patients) (1-6). Leishmania is common in Middle East including Iran. Visceral and cutaneous leishmaniasis has been reported in Iran, but there is no report of mucosal leishmaniasis until now in Iran. More recent reported incidence of cutaneous and visceral leishmaniasis in Iran estimates about 42.2 in 100000 and 0.3 in 100000, respectively (7). Leishmania tropica and major are the most common etiologic agents for cutaneous leishmaniasis in Iran. Leishmania tropica causes urban or dry type leishmaniasis (8). The face, neck and arms are the commonest site of involvement, although the location of the lesion in a covered area such as the shins has been reported in Iran. There are several reports of atypical cutaneous presentation such as paronychial, whitlow, lid, scar, palmoplantar and chancriform in immunocompetent patients (9). On the other hand, according to altered or over expressed immune host responses, various atypical pictures may occur that cause delay in diagnosis, like verrucose, psoriasiform, erysipeloid, zosteriform, mycetomatous, DLE-like, squamous cell carcinoma-like and eczematous morphologies (10-13).
2. Case Description
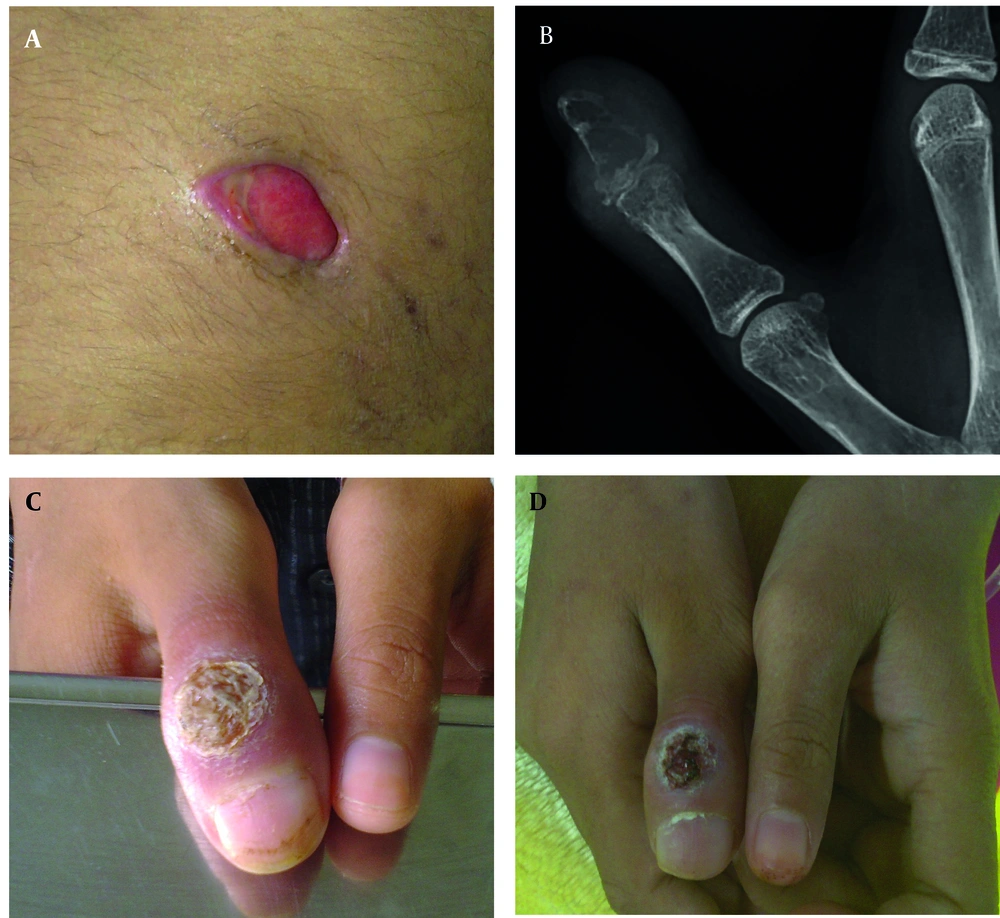
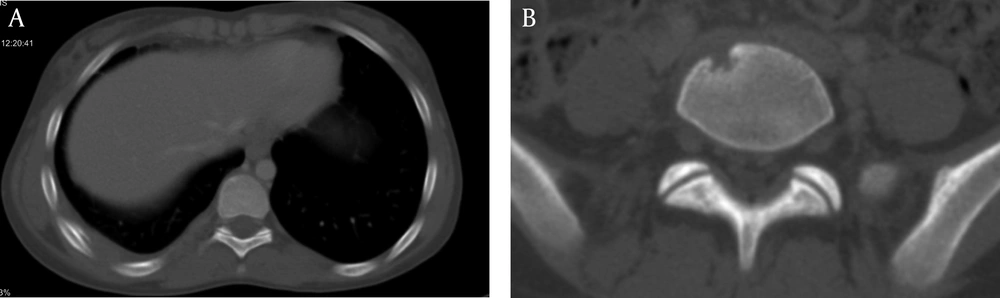
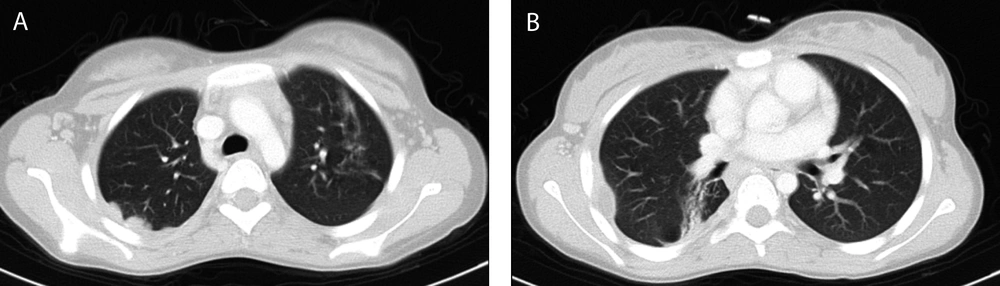
A 13 years-old girl admitted in surgery ward for debridement of chronic fistulating wound (Figure 1A). A small soft-tissue mass on her right subscapular region developed 4 months ago. It was not painful and had been enlarged gradually until reached about 10 × 10 cm after 3 months and then fistulated over time. No fever was mentioned during the course of her illness. Anti-leishmania antibody (ELISA) was checked 1 week before admission which was negative. In her physical exam: a 1.5 × 1.5 cm vegetated, dry ulcer on dorsal aspect of her first proximal interphalangial joint was detected, which was discovered 11 months ago (Figure 1C). In first work up, she had WBC: 7900 cell/mm3 (neutrophil: 83%, lymphocyte: 15%) and normal biochemistry. Additional investigations showed normal immunoglobulin profile and negative anti-HIV antibody titer. Wound draining fluid was sent for gram, and acid fast stain, aerobic and anaerobic culture and cytology (malignant cells) which all were negative, except for Giemsa staining which showed leishman bodies in the high-power field of microscopic examination. In histopathologic examination of subscapular ulcer, wound debridement showed necrotizing granulomatous change. The biopsy specimen acid fast bacili staining was negative for Mycobacterium tuberculosis. Periodic acid-schiff (PAS) with diastases was also negative for fungal elements. Additionally, pathology blocks were sent to the national research institute of tuberculosis and lung disease (NRITLD) to confirm the diagnosis; simple and nested PCR results were negative for Mycobacterium tuberculosis. No malignancy found and Zeihl Nelseen and Giemsa stains were also negative for acid fast bacili. The PPD injection site had a 20 mm induration size after 48 hours. More detailed history revealed that her stepmother received anti-tuberculosis treatment 5 to 6 years ago. She still had cough and evidence of pulmonary involvement in CXR and CT scan; so anti tuberculosis treatment was begun again. Treatment with a low dose Amphotricin-B began 1 day after admission in pediatric infectious ward. In bone x-ray of her hand a lytic expansile lesion was seen in distal phalanx of thumb with cortical irregularity associated with surrounding soft tissue swelling (Figure 1B). Spiral CT scan of chest showed enlarged lymph nodes in right and left axillary regions, nodular pleural thickening, soft tissue swelling and subcutaneous emphysema and a hypodens collection (16 × 10 mm) at the right chest wall with concomitant periosteal reaction in adjacent ribs (Figure 2). Sub-segmental consolidation with bronchial dilatation was seen in superior segment of the right lower lob (RLL). Parenchymal band or area of fibrosis was seen in the left upper lob (LUL). Spiral CT scan of the lumbar spine without IV contrast, demonstrated lytic area in inferior, right anterior aspect of L5 (Figure 3). A 6-month, four-drug initial regimen (6 months of Isoniazid and Rifampicin supplemented in the first 2 months with Pyrazinamide and Ethambutol) used for our patient. Amphotricin-B continued for 3 weeks. Throughout treatment the thumb ulcer became smaller and healed slowly (Figure 1D). Patient monitored until the lesions substantially resolved. Thereafter, patient warned about relapse.
3. Discussion
However, soft tissue involvement can be attributing to leishmanisis but rib, dactylitis and vertebral involvement may not explain exclusively by leishmanisis. On the other hand, pulmonary involvement as pleural thickening, pleural based nodule and parenchymal involvement in our patient in combination with bone involvement could not describe only with leishmanisis because pulmonary parenchymal involvement is considered as a rare complication of leishmaniasis and is mainly described in visceral leishmaniasis (VL) in the immunocompromised patient (14, 15). Additionally, bone involvement had not been reported previously in the literature except rarely in diffuse cutaneous leishmaniasis (9, 16). According to these reasons other co-infections considered for patient. We investigate to rule out tuberculosis, actinomycosis, nocardiasis and fungal infection as potential agents which justify the clinical picture of patient. Further investigation revealed positive past history of anti-tuberculosis treatment in her stepmother and interestingly, detection of active pulmonary parenchymal involvement in her stepmother. Positive PPD test, recent exposure and pulmonary involvement accompanied with dactylitis, rib and vertebral involvement in the absence of microbiological evidence of other possible causes, led us to start anti-tuberculosis treatment for patient as a probable case of tuberculosis according to WHO criteria (17).
We also investigated whether any predisposing factors such as primary or secondary immunodeficiency syndrome to find any explanation for this co-infection and positive interaction between tuberculosis and leishmaniasis.
Our advanced immunological screening tests (including peripheral blood flowcytometry, immunoglobulin profile study and NBT test, we could not check gamma loop assay because of limited accessibility to this test) did not reveal any evidence of primary or secondary common immunodeficiency disorders. Leishmaniasis and tuberculosis both are caused by intracellular pathogens whose development depends on impaired cell-mediated immunity (18). This Co-infection previously detected in specific population such as in patients with primary immunodeficiency as well as secondary immunodeficiency (especially in HIV positive patients) (1, 3-6, 19). On the other hand, poverty, overcrowding, malnutrition, illiteracy, and poor domestic conditions facilitate the growth of these diseases (1).
According to known tuberculosis infection interaction with cellular immunity and apoptosis-inhibiting effects, susceptibility to these organisms could be explained with gamma interferon independent mechanism (for example, alteration of Fas and Fas ligand expression and CD8 cytotoxic T cells dysregulation) (20-22). IL-4 has a significant role in inhibition of apoptosis and diversion of cell death to necrosis. This role was investigated in a study by Abebe et al. in a culture of infected human monocytic cell line THP-1 with virulent mycobacteria as compared with virulent strains. M. tuberculosis infection reduced apoptosis and either M. tuberculosis infection alone or with IL-4 treatment led to an increase ratio of cell death by necrosis compared to apoptosis (23). In contrast to apoptosis, inflammatory response is usually elicited in necrotic cell death and thus to releasing bacteria from necrotic cell and in the presence of inflammation and cytokine aggregation re-infection of adjacent cells will happen. In this situation, progression of latent leishmanial infection to clinical leishmaniasis can be provoked and vice versa.
A large study in multi-case families of tuberculosis, leprosy and leishmaniasis in northeastern Brazil showed genetic susceptibility to intra-macrophage pathogens. TB susceptibility on locus 17q11.2-q12, was found to be associated with susceptibility to leishmaniasis (24). Mutations in the human natural resistance–associated macrophage protein 1 (NRAMP1) gene also appear to play a major role in the innate susceptibility to tuberculosis, leprosy, and leishmaniasis (25), and so more genetic investigation is required.
Our theory may explain these unique phenomena in some imbalance between TH1 and TH2 host responses, which convert predominant TH1 responses in a low parasitic cutaneous form of leishmaniasis to opposite spectrum with predominance of TH2 responses as a consequence of co-infection with tuberculosis.