1. Context
Tuberculosis (TB) is a serious cause of morbidity and mortality all over the world (1). After HIV/AIDS, TB causes the greatest number of deaths due to a single infectious agent. According to released data 8.6 million people are infected by TB and 1.3 million die from TB. Most deaths related to TB (95%) occur in poor and middle-income countries; also TB is one of the three causes of death among women aged 15 to 44 years of age. According to statistics, 530000 children became ill with TB and 74000 HIV-negative children died from TB in 2012. People who are living with HIV are more exposed to death because of TB and combination of TB and HIV (2). Nowadays incidence and mortality from TB is at a steady rate, which is the result of governmental and non-governmental organization efforts and investments in the last decades in order to control this infection (3). To achieve the United Nations Millennium Goals of eliminating the disease by 2050, diagnosis and treatment of this active disease with new approaches to reduce this vast reservoir of latent tuberculosis infection (LTBI) is vital (4). An important strategy for TB control is utilization of rapid, accurate, and inexpensive ways for detection of active TB. Owing to the fact that diagnosis of LTBI has a major role in controlling of tuberculosis, it is highly vital to find the best diagnostic strategies for LTBI. In the present paper, we reviewed the latest guidelines and articles by using a comprehensive search of reliable data-bases such as PubMed and ISI on diagnosis and treatment of latent tuberculosis.
2. Purposeful Detection of Tuberculosis
Among high-risk groups, TB testing must be performed in order to treat LTBI. Once infection with M. tuberculosis is detected, control and evaluation of LTBI in high-risk groups for developing TB disease becomes highly important, hence targeted testing is essential and can prevent the spread of TB and control disease progression. In this way unfocused population-based testing is avoided as it is neither cost-effective nor useful and leads to unnecessary treatment (5).
Overall, TB afflicted patients belong to two major groups:
A) People who are at risk of exposure to TB patients including:
1) Close contact with TB afflicted patients
2) Immigrants from TB endemic regions
3) People who are working in institutions or hospitals that care for TB, nursing homes, or residential facilities for patients with HIV infection/AIDS
B) People with specific conditions who are at risk of progression from LTBI to TB disease:
1) HIV infected cases
2) Injection drug abusers
3) Those with radiographic evidence of prior healed TB
4) Those with low body weight (10% below ideal)
5) Patients with other medical conditions such as:
Silicosis, diabetes mellitus, chronic renal failure or on hemodialysis, gastrectomy, jejunoileal bypass, solid organ transplant, head and neck cancer
6) Recent tuberculin skin test (TST) converters (that is, people with baseline testing results who have an increase of 10 mm or more in the size of the TST reaction within a two-year period)
7) Infants and children under the age of five who have a positive TB test result (6). Note, the risk of progression is greatest in the first 1 or 2 years after infection (6).
3. Detection of Latent Tuberculosis Infection
Medical Records, TST or interferon gamma release assay (IGRA) results, chest radiograph, physical examination and sputum examinations in certain circumstances can be used for detection of LTBI. Before treatment of LTBI, the existence of TB disease must be excluded since inadequate treatment and development of drug resistance can lead to failure of treatment (6).
4. Latent Tuberculosis Infection and Disease Differentiation
4.1. Latent Tuberculosis Infection
Finding no symptoms and physical evidence of TB
Usually positive results for TST or IGRA tests
Normal Chest radiography
Negative respiratory smear and culture
Proper treatment of LTBI can prevent TB disease
4.2. Tuberculosis Disease
Fever, cough, chest pain, weight loss, night sweats, hemoptysis, fatigue, and decreased appetite are common symptoms of TB disease.
4.2.1. Positive Results for TST or IGRA Tests
Chest radiographs are abnormal in most cases of TB disease, however in patients with immunosuppression or extra-pulmonary disease the chest radiograph is normal.
Smear or culture of respiratory specimens is usually positive, except with extra-pulmonary disease or minimal or early pulmonary disease.
Transmission of TB bacteria to others requires treatment of TB diseases patients. Use of diagnostic tests for LTBI among individuals and populations at low risk for infection with M. tuberculosis is highly recommended. Testing is sometimes done to meet administrative or legal requirements for groups who are not considered to have an increased possibility of infection in the absence of other factors cited above, such as people meeting entrance requirements for certain schools and workplaces, despite Center of for Disease Control CDC recommendations .
5. Diagnosis of Latent Tuberculosis Infection
In an individual patient there is no way to directly detect the presence of latent Mycobacterium tuberculosis. Measurement of host immune responses as a surrogate for the presence of viable bacteria is the key for the assessment of latent infection (7).
5.1. Tuberculin Skin Testing
Tuberculin skin testing was the only diagnostic method for detecting LTBI until the beginning of this century. This method is based on a delayed hypersensitivity response which occurs when individuals contaminated with M. tuberculosis are exposed to certain antigenic components present in extracts of culture filtrates, “tuberculin”. T cells are sensitized by primary contamination and they are recruited to the skin where the tuberculin had been injected and release lymphokines in this type of infection. Local induration of the skin through local vasodilatation, edema, fibrin deposition, and recruitment of other inflammatory cells to the area are the results of the injection. A positive reaction can be accepted when the size of induration is greater than 5 mm, while various cut-off sizes should be considered (8).
5.1.1. Amplifying Phenomenon
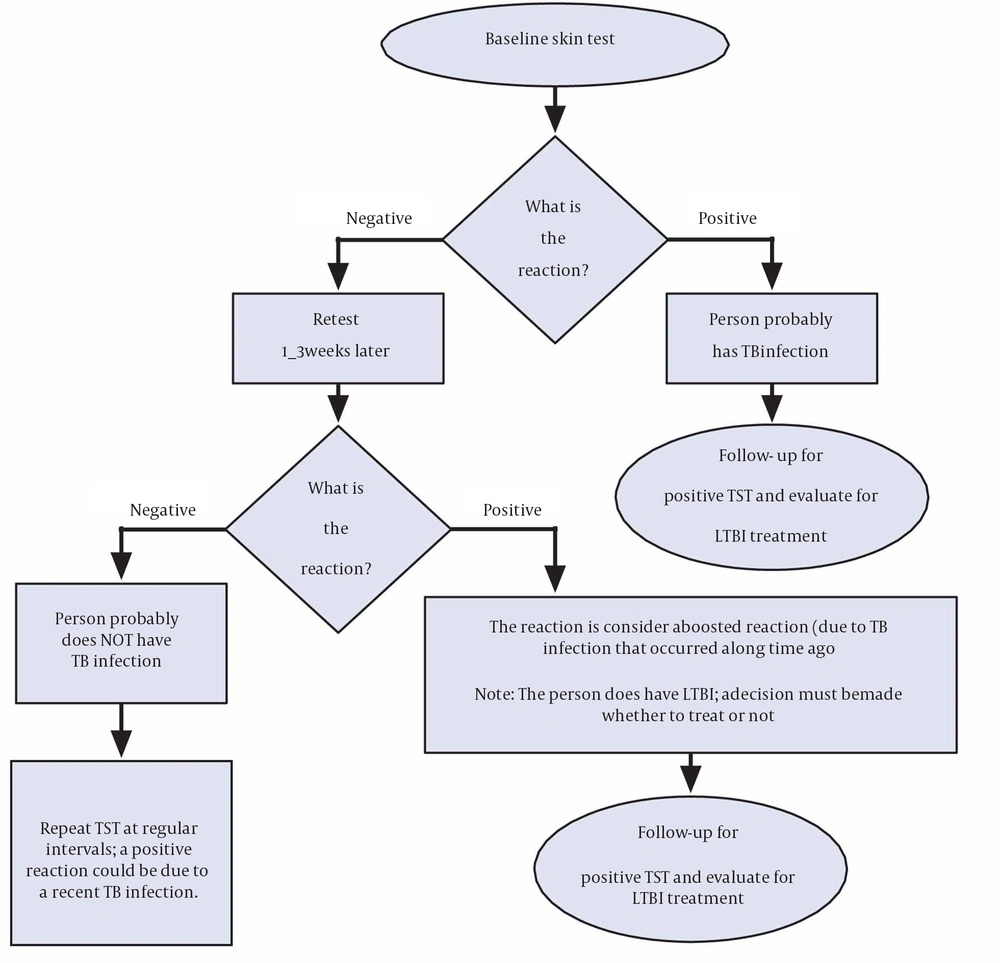
After many years have passed since a patient has become infected with M. tuberculosis, the reaction with TST may become negative. Repeated positive reaction with TST is representative of reaction to the test. This is commonly referred to as the “booster phenomenon” and may incorrectly be interpreted as a skin test conversion (going from negative to positive). For this purpose the “two-step method” is suggested at the time of initial testing and for people who may be tested periodically (9) (Figure 1). There is no need for a second test when IGRAs are used for serial testing, since amplifying does not occur (6).
5.1.2. Tuberculin Skin Testing Limitations
1) Malnutrition, severe TB diseases and immunodeficiency diseases such as HIV can reduce TST sensitivity.
2) In places where non-tuberculous mycobacteria (NTM) are prevalent and in populations who have received Bacille Camette-Gurin (BCG) vaccination after infancy, decreased TST specificity might occur, however the effect of BCG vaccination on TST reactions is variable after 10 years or more, if vaccination is administered during infancy (10).
3) Two health care visits are needed for completing the TST, one for tuberculin injection and the second visit for measurement of induration, which results in loss of reading in approximately 10% of cases (11).
4) Intensity of diversity in measurement of reaction size persist which can be successfully reduced by adequate training (12, 13).
5) Remote infection, which has a lower risk of progression to disease, cannot be distinguished by one positive TST result (14). Tuberculin skin testing reversions are more common in older adults; estimated at 8% per year (15).
6) When additional tests become un-interpretable, it means that a serial TST has no place in monitoring treatment responses. Repeated injections of tuberculin are known to elicit the amplifier phenomenon, also a two-step tuberculin test is recommended at the time of first testing, in situations where serial testing is indicated, in order to reach this reason (16, 17). In low and medium-income countries with a high-TB incidence, diagnosis of LTBI, detection and treatment of active TB is difficult and should be a priority; however some of these countries could invest in detection and treatment of LTBI if an easier test was available (18, 19).
It seems that TST benefits from many advantages. Clinical applications of TST are widely studied and it has been widely used during the past century. For indication of Isoniazid Preventive Therapy (IPT) for different ages and risk groups cut-off points have been established (20). More importantly, the benefit of IPT in groups with a positive TST has been extensively proven, as has been the lack of benefit of IPT in TST-negative subjects (20, 21). Although the role of TST alone for evaluating the risk of progression to disease is limited, the main application of TST is to detect LTBI. For evaluation of risk of disease this testing method must be interpreted in light of the clinical situation and epidemiological history (6).
5.2. Interferon-Gamma Release Assays (IGRAs)
By measuring the immune response to TB proteins in whoe blood, IGRAs are used to determine if a person is infected with M. tuberculosis. Antigens derived from M. tuberculosis are mixed with peptides to stimulate immune systemresponse. White blood cells recognize the simulated antigens and release interfereon gamma (IFN-γ) in a person infected with M. tuberculosis (22). Two U.S Food and drug administration approved IGRAs are commercially are available in the United States:
QuantiFERON ®-TB Gold-in-Tube test (QFT-GIT)
T-SPOT ®.TB test
5.2.1. Advantages of IGRAs Include the Following
1. A single patient visit to conduct the test
2. No Booster phenomenon
3. A laboratory test not affected by health care worker's perception or bias
4. Results can be available within 24 hours
5. Unaffected by BCG and most environmental mycobacteria
5.2.2. Limitations of Interferon-Gamma Release Assays Include the Following
Once blood samples are collected, they should be processed within 8-30 hours.
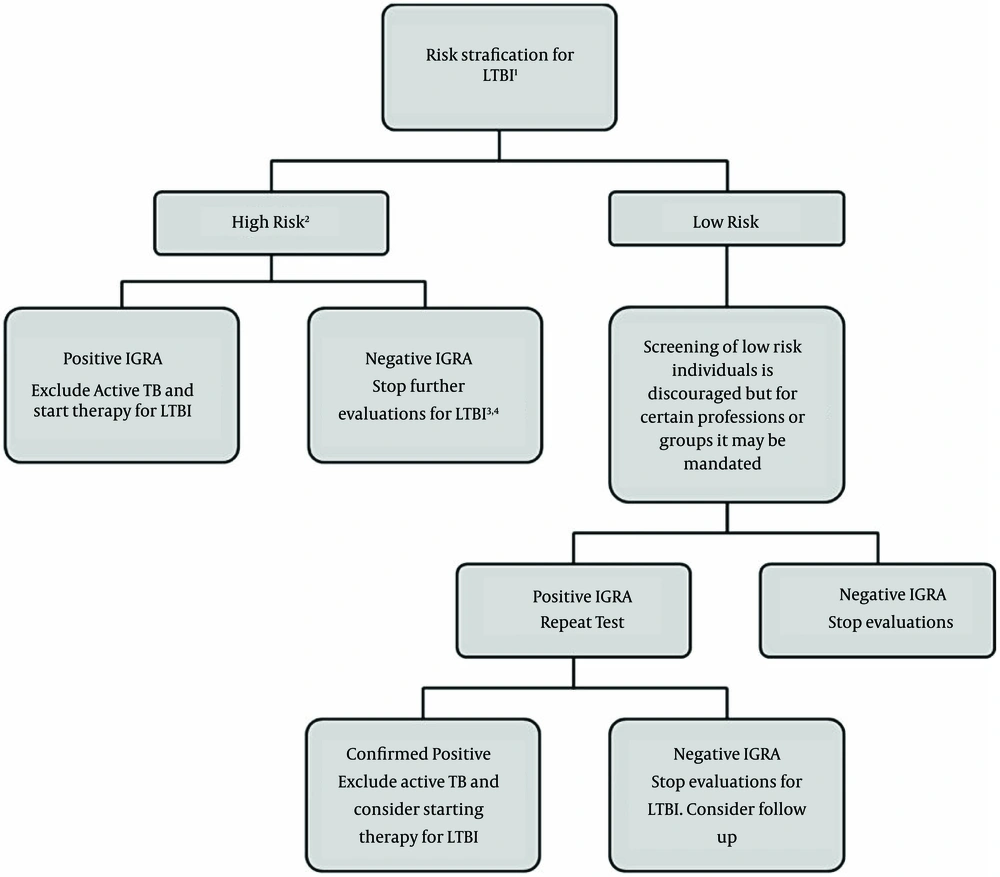
There is limited data on the use of this method in groups such as children younger than five years of age, people recently exposed to TB, immunocompromised individuals, and those who are required be tested repeatedly (serial testing) (22). An algorithm for LTBI screening with IGRAs is presented in Figure 2 (23).
1) Risk stratification is based on the criteria established by the CDC. 2) After an exposure, consider waiting at least 4-7 weeks before testing. 3) In certain groups (ie. close contacts of patients with active TB), consider excluding active TB and treating for LTBI despite negative IGRA results. 4) IGRAs have lower sensitivity in children and immunocompromised patients.
5.2.3. Interferon-Gamma Release Assay for Special Populations
Distinct subpopulations such as children, patients living with HIV/AIDS (PLWHA), other immunosuppressed patients (those with renal disease or using tumor necrosis factor-alpha inhibitors), and healthcare workers can prove the accuracy of IGRA tests (24-31).
5.2.3.1. A) Immunosuppressed Patients
The sensitivity of IGRA tests has been examined for patients living with HIV/AIDS (PLWHA) and those with active TB. Sensitivities of both TST and IGRA tests were lower in PLWHA because both require an adequate immune response; this was indicated in 16 from 18 studies. The T-SPOT.TB test has a pooled sensitivity of 76% while QFT-GIT has a pooled sensitivity of 60%. The Pulmonary Medicine 5 seems to be slightly less affected by immunosuppression (19). However, only five studies compared their sensitivity head to head (32-36), with only one study showing Quantiferon GIT QFT-GIT with a greater sensitivity than T-SPOT.TB in PLWHA (36). A prospective study from Iran (by Mardani et al.) compared TST and Quantiferon TB gold in 50 HIV positive patients. This study showed no association between a positive TST and receiving highly active anti-retroviral therapy (HAART) or absolute CD4 count (29). Similarly, the association between QFT-G results and receiving HAART or CD4 counts was not significant. Although TST results were not significantly different in patients with CD4 < 200 versus CD4 > 200, the association between QFT-G results and CD4 cutoff of 200, reached statistical significance (29). Also the role of IGRA in patients with autoimmune disease has been analyzed by 14 studies (37-51); 10 of which were performed in countries where BCG vaccination is not routinely used (37-39, 42-44, 46, 47, 49, 50). Immunosuppressive therapy was variable or not yet started in most studies that included a small number of patients; also very few were conducted in countries with high-TB prevalence (51). In a study from Iran by Mardani et al. results of TST and Quantiferon TB Gold test were compared for lung and heart transplant patients. Of the 55 patients included in the study, three (5%) had positive tuberculin skin test results, and 11 (20%) had positive QuantiFERON-TB Gold In-Tube test results. The positivity for QuantiFERON-TB Gold In-Tube test was greater than the positivity for the tuberculin skin test, and QuantiFERON-TB Gold In-Tube test more accurately determined the risk for latent tuberculosis infection (31). Overall, there is no study to Sum up the efficacy of IGRA tests for transplant patients under immunosuppressive therapy. When interpreting conversion and reversions in PLWHA, special caution must be taken. Declining immunity can lead to reversion and should alert the physician. In contrast, naive TST negative individuals with advanced HIV infection (CD4+ cell count < 200/μL) started on HAART for LTBI/TB treatment, should be retested for LTBI once they achieve CD4+ cell counts > 200/μL (52).
5.2.3.2. B) Children
The sensitivity and specificity of IGRA in older children exposed to index cases are similar compared to adults, as long as they are not infected by HIV; although a few studies have shown a lower sensitivity for IGRA tests in very young children (25, 53, 54). The lower sensitivity found for both TST and IGRAs among very young children might be explained by immune immaturity or having a population with underlying conditions that may interfere with immunity, such as co-infections with helminthes and malnutrition; although BCG is not expected to interfere with IGRAs (55). It should be concluded that in all children with active TB, sensitivity and specificity were similar for TST, QFT-G/QFT-GIT (QFT), and T-SPOT.TB (25).
5.2.3.3. C) Highly Exposed Populations and Serial Testing
Health care workers and prisoners, as well as patients at high risk of disease should be considered as highly exposed groups, such as PLWHA, and they should be screened for LTBI every 6 to 12 months. Boosting of IGRA responses is not seen if QFTGIT or T-SPOT.TB is done within three days of performing the TST, although it has been reported that tuberculin injection may also result this effect (56, 57). Furthermore, IGRA tests may be repeated, regardless of their previous results, unlike TST, which should only be repeated if previously negative. However, serial testing of subjects not undergoing treatment has indicated that there are high rates of spontaneous reversions and conversions, although it is always harder to know if conversion is spontaneous or a consequence of real TB infections (58, 59). Nonspecific infections and vaccines, laboratorial technique variability (such as blood withdrawal preparation), delay to and time of incubation, and the kit being used are other possible explanations for a conversion. The predictive value of IGRA (QFT) conversion for the development of TB disease is still controversial, and fluctuations in IFN-γ responses among serially tested individuals reported in longitudinal studies remain unexplained and nonspecific. Although there is some evidence that QFT-GIT conversion is related to a greater risk of progression to TB (60), spontaneous reversions are more likely to occur in subjects with borderline results, (56, 61). Such cases are interpreted as self-clearance of infection, yet there is not enough evidence for this conclusion (52, 62). Serial testing with QFT-GIT and T.SPOT-TB should be considered unreliable and is not recommended at least until the conversion and reversion phenomena are better understood.
6. Selecting a Test to Detect Tuberculosis Infection
However there are certain situations where results from both tests may be useful, although routine testing with both TST and IGRAs is NOT recommended.
For the following groups IGRAs is the preferred method of testing:
1. People who have poor rates of return for TST reading and interpretation such as homeless individuals
2. People who have been immunized by BCG vaccination
Otherwise without preference for other groups that are tested for LTBI, TST or IGRA can be used (6).
6.1. Guidelines From Professional Societies
There are screening guideline for latent tuberculosis infection in the United States, Canada and the United Kingdom (Table 1). Although there are various recommendations yet they all endorse the use of IGRAs in some circumstances, with U.S. guidelines advising that IGRAs can be used interchangeably with the tuberculin skin test in most circumstances. The guidelines are likely to evolve as more data on IGRAs become available.
Since IGRAs became available commercially, there have been several publications on its accuracy, predictive value, cost-effectiveness, and its indications. Also these tests have been incorporated in national guidelines of many high-income countries (6).
Recently the World Health Organization (WHO) published a set of recommendations for low- and medium-income countries (LMIC) (6); more extrapolation in middle-income settings with high background TB infection is needed since most IGRA studies have been done in high-income countries. Moreover, IGRA performance differs in high-versus low-TB and HIV incidence settings, with relatively lower sensitivity in high-burden settings which is suggested by systematic reviews. Cost is one of the most important aspects against their use in LMICs.
| Risk Group | US Guideline | Canadian Guideline | UK Guideline |
|---|---|---|---|
| Close contacts of persons with infectious TB | TST or IGRA, but not both | TST, with IGRA to confirm positive TST | TST, with IGRA to confirm positive TST |
| Persons who may not return for TST reading because of circumstances (e.g. homelessness or injection-drug use) or logistic difficulties | IGRA preferred | no specific recommendation | GRA preferred |
| Immunosuppressed persons (e.g. those infected with HIV or receiving treatment with prednisone or TNF-α inhibitor) | TST or IGRA; use both if first is negative and suspicion is high | TST, followed by IGRA if TST is negative | TST or IGRA |
| Foreign-born persons | screening only for those who have immigrated in past 5 yearss; use TST or IGRA, but not both | screening only for those < 15 years old who have immigrated in past 2 years; use TST, with IGRA to confirm positive TST | screening for new immigrants only; use TST with IGRA to confirm positive TST for those 5-15 years of age and IGRA for those 16-35 years of age |
| BCG vaccine recipients (if they belong to another risk group) | IGRA preferred | no specific recommendation | TST or IGRA |
| Health care workers (screening program) | TST or IGRA, but not both | TST preferred | TST or IGRA, depending on specific circumstances |
| Children < 5 year old | TST preferred | no specific recommendation | TST preferred |
| Other risk groups | TST or IGRA, but not both | TST, with IGRA to confirm positive TST | TST, with IGRA to confirm positive TST |
aThe sources for the US, Canadian and UK guidelines, respectively, are as follows: the Centers for Disease Control and Prevention, the Public Health Agency of Canada; and the UK; National Institute for Health and Clinical Excellence. BCG denotes Bacille Calmette–Guerin, HIV human immunodeficiency virus, IGRA interferon-γ release assay, and TST tuberculin skin test.
6.2. Special Considerations in Testing for Tuberculosis Infection
6.2.1. Bacille Camette-Gurin Vaccine
Confusion often results from the effect of BCG vaccine on TST results. Although periodic skin testing may prolong (boost) reactivity in vaccinated people, TST reactivity caused by BCG vaccine generally decreases with the passage of time. If people react to TST, those with a history of BCG vaccination can be tested and treated for LTBI. Based on risk stratification, regardless of BCG vaccination history, TST reactions should be interpreted. Specific antigens that do not cross react with BCG are used by IGRAs, thus positive reactions in BCG recipients do not occur with IGRA (6).
6.2.2. HIV Infection
For those with both LTBI and untreated HIV infection, the risk of progression from LTBI to TB disease is 7% to 10% each year. Patients with LTBI who are not suffering from HIV, have a 10% risk over their lifetime. Thus, the risk of progression to TB disease is 10 times greater for those who are HIV infected. Although this risk is still higher than that of HIV-negative individuals with LTBI but antiretroviral therapy for HIV reduces this risk. As soon as HIV status of HIV infected patients becomes known, they should be tested for LTBI. A negative TST or IGRA result does not exclude LTBI as they may have a compromised ability to react to tests for TB infection. Annual testing should be considered for HIV-infected individuals who are TST or IGRA negative on initial evaluation, and who are at risk of exposure to M. tuberculosis. The usefulness of anergy testing for HIV-infected individuals or others has not been demonstrated; therefore, it is not recommended. After the initiation of antiretroviral therapy (ART), repeat testing for LTBI is recommended for HIV-infected individuals previously known to have negative TST or IGRA results. This is because the immune response may be restored by ART (63).
7. Contacts
1. For contacts of a person with infectious TB disease, retesting in 8-10 weeks after the end of exposure is indicated when the initial TST or IGRA result is negative. In contact investigations, retesting is not called two-step testing. The second test is needed to determine if infection occurred, but was too recent to be detected at the time of the first test.
2. Children under the age of five years and immunosuppressed individuals (e.g. HIV infected) who have negative TST or IGRA results should have a chest radiograph. If the chest radiograph is normal, treatment should be started for LTBI and another TST or IGRA should be performed 8-10 weeks after contact has ended.
3. If a repeat TST or IGRA result is positive, treatment should be continued. If this is negative, treatment can usually be discontinued.
4. If testing is repeated, the same type of test (TST or IGRA) should be used (6).
8. Pregnancy
1. TST is both safe and reliable throughout the course of pregnancy.
2. Only test if specific risk factors are present for acquiring LTBI or for progression of LTBI to TB disease
3. If a TST or IGRA reaction is positive, obtain a chest radiograph using proper shielding (64).
9. Discussion
In conclusion, although TST is a simple, reliable and available method but in some cases IGRA provides a better yield which helps in making rational decisions for treatment of LTBI. A coordinated strategy will be required to effectively tackle the reservoir of latent infection. Improved data are needed to more accurately estimate the scale of the problem and quantify the number of new infections occurring each year, and a redoubling of effort will be required to reduce this as far as possible by implementing currently recommended interventions. However, in order for widespread treatment of latent TB to be acceptable to the public, healthcare providers and policy makers, need to inform the general population. Major advances on the currently available diagnostic and interventional tools are required to control LTBI. Progress in identifying who is most likely to react and how this occurs will assist the development of more predictive diagnostic tests allowing interventions to be focused on those that will benefit most.