1. Background
A. baumannii, K. pneumoniae, and P. aeruginosa are considered as a group of three pathogens responsible for the most of multidrug-resistance (MDR) infections in hospital intensive care units (ICUs) around the globe and are the major challenge for physicians due to lack of effective treatment options especially in case of carbapenem resistant strains (1, 2).
Zinc oxide (ZnO) and titanium dioxide (TiO2) nanoparticles (NPs) are recently studied for their antimicrobial activity against MDR isolates of methicillin-resistant Staphylococcus aureus (MRSA) (3, 4). Reports suggested that covering of implants with nano-TiO2 stopped the adhesion and growth of bacteria sp., such as Streptococcus mutants, S. epidermis, and Escherichia coli, as well as preventing the inflammation (5, 6). TiO2 NPs is effective against many bacteria including spores of bacillus, which is the most resistant organism known (7). Furthermore, ZnO NPs exerted antibacterial activities against both Gram-positive and Gram-negative bacteria, such as major foodborne bacterial pathogens like E. coli O157:H7, Salmonella sp., Listeria monocytogenes, and S. aureus (8). It has been shown that ZnO, Ag, and Ag doped titanium dioxide (Ag-TiO2) had broad-spectrum antimicrobial activity against bacterial strains tested (9). SEM analysis revealed that administration of TiO2 NPs increases the bacterial cell volume causes changes in the cell membrane permeability, which led to cell lysis (10). ZnO NPs was relatively effective against pathogens such as K. pneumonia, L. monocytogenes, and Salmonella enteritidis (11).
Beside the nanometals, it has been reported that antibacterial peptides such as mastoparan-B (MP-B) and indolicidin are very effective for treating bacterial infections (12). MP-B is an amphiphilic alpha-helical cationic antimicrobial peptide isolated from the venom of hornet Vespa basalis (12). MP-B affects biological activity alone and in combination with clinically used antibiotics against Acinetobacter baumannii (13). Its chemical structure allows electrostatic interactions between the positively charged Lysine residues in MP-B and the anionic phospholipid of the cell membrane. MP-B demonstrated strong antimicrobial activities against both Gram-positive and Gram-negative pathogens (14). Investigations suggested two E. coli strains, S. xylosus and Citrobacter koseri at low dosages (0.1 mg/L), were sensitive to this compound (15). In another study, MP-B and its analogs were found to be safe to the probiotics after undergoing appropriate amino acid substitutions (16).
Indolicidin is a cationic antimicrobial peptide isolated from bovine neutrophils. It has the highest tryptophan content of any known protein (17). Despite the small size and unique composition, indolicidin is capable of killing Gram-negative bacteria by crossing the outer membrane and causing disruption of the cytoplasmic membrane channel formation (18). Jindal et al., (19) evaluated the microbicidal activity of indolicidin against S. aureus and E. coli as test organisms. E. coli was more susceptible to indolicidin.
2. Objectives
The objectives of this study were to evaluate the antibacterial activity and synergistic effects of the mastoparan-B, indolicidin, TiO2, and ZnO NPs against drug- resistant isolates of P. aeruginosa, A. baumannii, and K. pneumoniae isolated from a referral hospitals in Kerman, Iran.
3. Methods
3.1. Specimen Collection and Samples Sources
From March to August 2015, we isolated a total of 30 samples (10 each) of antibiotic resistant MDR strains of P. aeruginosa, K. pneumoniae, and A. baumannii from patients hospitalized in adult ICUs of two referral hospitals in Kerman, Iran. Duplicate samples were collected from each patient by a laboratory technician. The samples were taken from the tracheal aspirate, blood, or urine. They were initially inoculated on MacConkey, Citrimate, and Cysteine electrolyte deficient (CLED) agar medium and incubated for 24 h at 37 ºC. The individual colonies were then identified as P. aeruginosa, K. pneumoniae, and A. baumannii by biochemical characteristics and conventional diagnostic tests as described previously (20).
3.2. Antimicrobial Susceptibility Testing
The antibiotic sensitivity of each isolate to 17 antibiotics was determined by disk diffusion breakpoint method as described by the CLSI guideline (21). The zone of inhibition diameter surrounding each disk was measured and labeled as resistance, intermediate, and sensitive according to the manufacturer protocol (Mast group Ltd. UK). Susceptibility to tigecycline was classified based on the EUCAST criteria. The following antibiotics were used in this study with concentration µg cm-1, tigecycline (TIG, 15), ceftazidime (CAZ, 30), imipenem (IMP, 10), meropenem (MEM, 10), amikacin (AK, 30), cefoxitin (CFO, 30), Gentamicin (GM, 30), cefpirome (CPO, 30), fosfomycin (FO, 50), ciprofloxacin (CIP, 5), ceftizoxime (ZOX, 30), norfloxacin (NOR, 10), and cefepime (FEP, 30). E. coli ATCC 25922 was used as a control strain in an antimicrobial susceptibility study.
3.3. Compounds Sources
Synthetic nano-TiO2 (Sigma-Aldrich CAS number 1317-70-0) and nano-ZnO (Sigma-Aldrich CAS Number: 1314-13-2) were purchased from the nano-Sany Corporation Ltd., (Mashhad, Iran) with the average purity of 99.7% trace metals basis. The nanoparticles were added into 5 ml D/W containing 5% of polyethylene glycol (PEG). PEG was used to disperse the colloidal nanoparticles and was not inhibitory at this concentration. The nanoparticles were then homogenized after 30 min of incubation at 37 ºC by sonication at 100 w, 40 KHz (to disperse the nanoparticle), and the dispersed solutions were autoclaved at 121 ºC for 15 min and kept at 4 °C. The purity of nanometals was checked by scanning electron microscope (SEM) and X-ray diffraction (XRD) analysis. Similarly, mastoparan-B and indolicidin peptides were purchased from BIO BASIC Int., (Canada), with the purity of 99%. The peptides purity was further checked by HPLC and Mass spectroscopy by company.
3.4. Determination of Bactericidal Activity of Nanometals, and Synthetic Peptides
Broth microdilution method was used for determination of MIC and MBC tests according to the CLSI recommendations (22). Briefly, primary stock solution (2560 mg/L) of each nanoparticle and peptides was prepared and diluted in the concentrations ranged from 5 to 2560 mg/L (13). A total of 50 µL of 2-fold serial dilutions of peptides was added to 96-well microtiter plates containing 50 µL of sterile synthetic M9 medium as the source of carbon for metal oxide NPs and Luria -Bertani (LB) broth (Merck, Germany) for the peptides. We used synthetic M9 medium to avoid precipitation of nanometals with component of the complex medium. To this preparation, 50 µL of the bacterial sample at 1 × 106 CFU/mL was added to each well. Prior to this, the microplates containing two fold dilutions of nano-TiO2 was placed under UV light for 15 min with 10 cm distance from UV source (antimicrobial peptides did not expose to UV light). The trays were rolled with aluminum foil and placed in plastic bags to stop evaporation of water incubated at 37 ºC for 24 h and the turbidity was measured at 650 nm or by the naked eye. MIC was defined as the lowest concentration of the above compounds that inhibits the bacterial growth. Simultaneously, a positive control (standard bacteria) and negative control (without bacterium) were run along the tests. A loopful of MIC concentration (no visible growth) was then streaked on Muller-Hinton agar and checked for colonies after 24 h of incubation at 37 ºC. The number of colonies formed was calculated and considered as MBC. The MBC was defined as the lowest concentration of nano-TiO2, nano-ZnO, and antibacterial peptides causing at least 99.9% killing of the initial number of bacteria. The experiment was carried out in triplicate to confirm the genunity of the results.
3.5. Determination of Antimicrobial Synergism
Checkerboard titration assay was carried out to obtain the fractional inhibitory concentration (FIC) indexes of each antimicrobial compound as described before (23). For this purpose, different concentrations of nano-TiO2, nano-ZnO, and antimicrobial peptides at sub-MIC level were prepared. A total of 50 μL of chemically defined M9 medium plus 0.2% glucose (glucose was sterilized by filtration) was distributed into each microtiter well containing nanometals oxides and sterile LB broth medium for peptides. 50 μL of the first compound of the combination was serially diluted along the ordinate, while the second was diluted along the abscissa. 25 µL of each preparation was then mixed and placed in microtiter plate wells in different combinations. A total of 50 µL of inoculum (CFU/mL 1 × 106) was then added resulting in identical rows. The microplate containing nano-TiO2 was placed under UV light for 15 min prior to incubation and one well was kept as control. After 24 h of incubation at 37 ºC, the turbidity was measured as described in the above section. The results were expressed as the FIC, which was determined as follows:
FIC = ([A]/MICA) + ([B]/MICB)
MICA and MICB are the MICs of compound A and compound B alone and [A] and [B] are the MICs of A and B when in combination. The FIC index was interpreted as follows: FIC ≤ 0.5, synergistic effect; 0.5 < FIC ≤ 1, additive effect; 1 < FIC ≤ 2, indifferent effect. For calculating synergistic action, we used fractional FIC = 0.5 as borderline as described previously (23).
3.6. Time- Kill Curve Assay
Time-kill curve analyses of P. aeruginosa, K. pneumoniae, and A. baumannii was performed in dilutions that had the best FIC index in the checkerboard (24). In brief, 5 mL of broth was added into four sets of Falcon tubes containing 50 µL of bacterial samples at a 0.5 McFarland inoculum (106 CFU/mL). The cells were exposed to concentrations of MIC ×1, MIC ×2, MIC ×4, and MIC × 8 folds for above combinations and one set was kept untreated as a control. Bacterial growth was then estimating at different time intervals (0, 4, 7 and 24 hours). The lower limit of sensitivity of colony counts was 100 CFU/mL. The antimicrobial agents used were considered synergistic at the lowest concentration that reduced the original inoculum by 3 log10 CFU/mL (FIC = 0.05) for each of the indicated time.
4. Results
4.1. Antimicrobial Susceptibility
All the isolates were resistant to commonly used antibiotics as shown in Table 1. A. baumannii isolates were resistant to 11 antibiotics tested, including imipenem, meropenem, amikacin, cefoxitin, gentamicin, and cefpirome, however, all were sensitive to tigecycline, respectively. Overall, we observed that the number of A. baumannii isolates that exhibited resistance to different classes of antibiotics were more as compared to K. pneumoniae and P. aeruginosa. Furthermore, K. pneumoniae isolates were resistant to ceftazidime, amikacin, cefotaxime, cefoxitin, gentamicin, cefpirome, ciprofloxacin, and imipenem (Table 1).
| Antibiotics | K. pneumonia (No. of Isolates) | A. baumannii (No. of Isolates) | P. aeruginosa (No. of Isolates) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| R | I | S | R | I | S | R | I | S | |
| TIG | 30 | 0 | 70 | 0 | 10 | 90 | 50 | 10 | 40 |
| CAZ | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 |
| IMP | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 |
| MEM | 90 | 10 | 0 | 100 | 0 | 0 | 100 | 0 | 0 |
| AK | 100 | 0 | 0 | 90 | 0 | 10 | 70 | 0 | 30 |
| CFO | 100 | 0 | 0 | ND | ND | ND | ND | ND | ND |
| GM | 100 | 0 | 0 | 90 | 0 | 10 | 90 | 10 | 0 |
| CPO | 100 | 0 | 0 | 100 | 0 | 0 | 90 | 0 | 10 |
| FO | 20 | 20 | 60 | ND | ND | ND | ND | ND | ND |
| CIP | 70 | 10 | 20 | 100 | 0 | 0 | 100 | 0 | 0 |
| ZOX | 100 | 0 | 0 | ND | ND | ND | 100 | 0 | 0 |
| NOR | 60 | 20 | 20 | ND | ND | ND | ND | ND | ND |
| FEP | 80 | 20 | 0 | 100 | 0 | 0 | ND | ND | ND |
| AZT | ND | ND | ND | 100 | 0 | 0 | 90 | 0 | 10 |
| PTZ | ND | ND | ND | 100 | 0 | 0 | 80 | 10 | 10 |
Abbreviations: AK, amikacin; CAZ, ceftazidime; CFO, cefoxitin; CPO, cefpirome; CIP, ciprofloxacin; FO, Fosfomycin; FEP, cefepime; GM, gentamicin; TIG, tigecycline; IMP, imipenem; I, intermediate; MEM, meropenem; NOR, norfloxacin; ND, not determined; R, resistance; S, sensitive; ZOX, ceftizoxime.
aAntibiotic sensitivity was determined in Muller-Hinton agar with inoculum concentration 1 ×106 CFU/ml.
4.2. Compounds Characterization
SEM analyses provided evidence that titanium and zinc aggregates were composed of oxide nanoparticles, spherical shape with size ranges from 20 to 50 nm. Purity was determined by X-ray diffraction analyses at 60 Kev over the range of 20 - 80º. Similarly, peptides purities were checked by HPLC and Mass spectrometer. The data was provided by nano Sany Company.
4.3. Antibacterial Activity of the Mastoparan-B, Indolicidin, ZnO NPs and TiO2 NPs
MP-B induced broad-spectrum bactericidal effect on A. baumannii, K. pneumoniae, and P. aeruginosa MDR isolates tested and inhibited the growth at mean MIC concentration 4 ± 0.2 mg/L; in contrast, no growth inhibition was detected for TiO2 NPs (MIC ≥ 1280 ± 0.2 mg/L) (Table 2). We also screened peptides and nano metals for toxicity against P. aeruginosa isolates. All isolates were resistant to TiO2 NPs and showed MIC ≤ 2560 mg/L. We observed that P. aeruginosa was less susceptible to mastoparan and indolicidin as compared to A. baumannii and K. pneumoniae with MIC 64 mg/mL (Table 2). From a susceptibility point of view, A. baumannii was more susceptible to the above antimicrobial agents than other bacterial species tested.
| Isolate No. | Mastoparan-B | Indolicidin | Nano-Tio2 | Nano-ZnO | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| AB | ||||||||
| 1 | 4 | 4 | 16 | 16 | 640 | 640 | 80 | 80 |
| 2 | 4 | 4 | 8 | 32 | 640 | 640 | 80 | 80 |
| 3 | 4 | 8 | 16 | 16 | 320 | 320 | 40 | 80 |
| 4 | 4 | 4 | 32 | 32 | 640 | 640 | 160 | 320 |
| 5 | 4 | 4 | 16 | 16 | 640 | 640 | 320 | 320 |
| 6 | 16 | 32 | 32 | 64 | 1280 | 1280 | 80 | 80 |
| 7 | 4 | 4 | 16 | 16 | 1280 | 1280 | 80 | 80 |
| 8 | 4 | 4 | 32 | 32 | 640 | 1280 | 320 | 320 |
| 9 | 2 | 2 | 8 | 16 | 320 | 320 | 40 | 80 |
| 10 | 4 | 4 | 16 | 32 | 640 | 640 | 80 | 80 |
| KP | ||||||||
| 1 | 8 | 8 | 64 | 64 | 1280 | 1280 | 160 | 160 |
| 2 | 8 | 16 | 32 | 32 | 1280 | 1280 | 160 | 160 |
| 3 | 16 | 16 | 32 | 64 | 2560 | 2560 | 640 | 640 |
| 4 | 8 | 8 | 32 | 32 | 1280 | 1280 | 160 | 160 |
| 5 | 16 | 32 | 64 | 64 | 640 | 1280 | 320 | 320 |
| 6 | 8 | 8 | 32 | 32 | 1280 | 1280 | 80 | 160 |
| 7 | 8 | 8 | 32 | 64 | 1280 | 1280 | 320 | 320 |
| 8 | 8 | 8 | 64 | 64 | 2560 | 2560 | 640 | 640 |
| 9 | 4 | 4 | 32 | 32 | 1280 | 1280 | 160 | 160 |
| 10 | 4 | 4 | 32 | 32 | 640 | 640 | 160 | 160 |
| PS | ||||||||
| 1 | 32 | 32 | 64 | 128 | ≥ 2560 | ND | 2560 | 2560 |
| 2 | 32 | 32 | 64 | 64 | ≥ 2560 | ND | 2560 | 2560 |
| 3 | 64 | 64 | 128 | 128 | ≥ 2560 | ND | ≥2560 | ND |
| 4 | 32 | 64 | 64 | 64 | ≥ 2560 | ND | 1280 | 1280 |
| 5 | 64 | 64 | 128 | 128 | ≥ 2560 | ND | 2560 | 2560 |
| 6 | 128 | 128 | 128 | 256 | ≥ 2560 | ND | ≥2560 | ND |
| 7 | 32 | 32 | 64 | 64 | ≥ 2560 | ND | 2560 | 2560 |
| 8 | 32 | 32 | 128 | 128 | ≥ 2560 | ND | ≥2560 | ND |
| 9 | 64 | 64 | 128 | 128 | ≥ 2560 | ND | 1280 | 1280 |
| 10 | 32 | 64 | 64 | 64 | ≥ 2560 | ND | 2560 | 2560 |
Abbreviations: AB, A. baumannii; KP, K. pneumonia; MIC, mg/mL; MBC, mg/mL; ND, not determined; PS, P. aeruginosa.
aSterile M9 medium containing glucose as the source of carbon was used for metal oxide NPs and LB broth was used for peptides MIC determinations. MICs were performed in triplicate with a standard deviation of ± 0.2. Bacterial sample was used at 1 × 106 CFU/mL.
4.4. Synergism and Time-Kill Curves Analysis
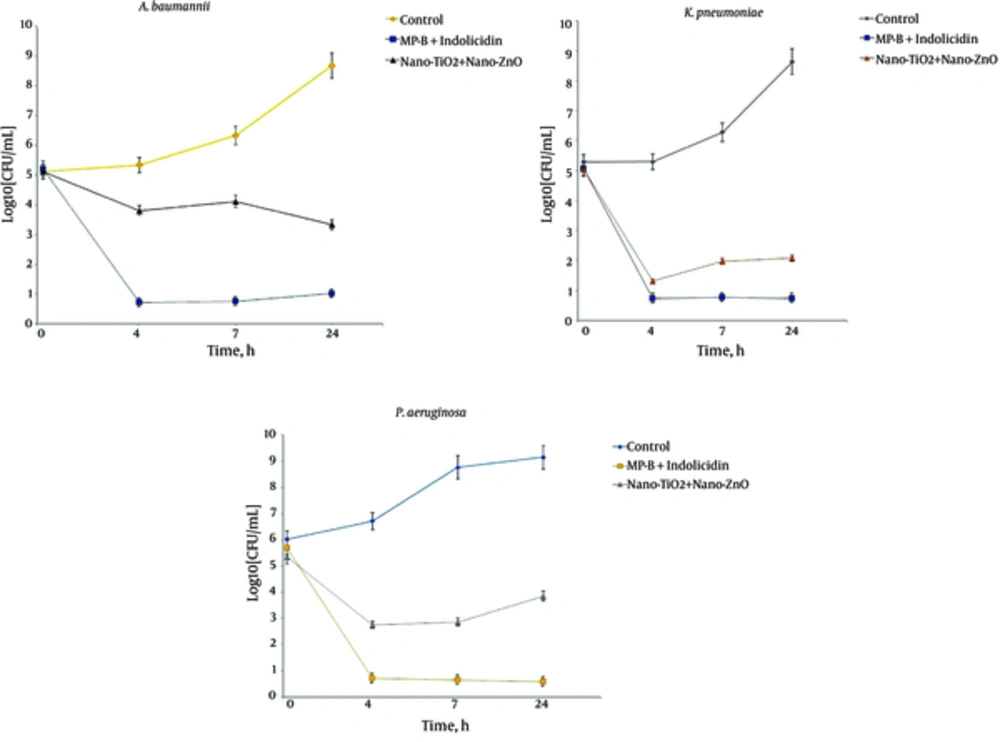
Checkerboard titration method revealed that both the nano-TiO2 and nano-ZnO NPs had an additive but not synergistic effect against A. baumannii and K. pneumoniae isolates (FIC = 1 ± 0.2), while P. aeruginosa isolates were indifference to the combination of these compounds (FIC ≤ 2 ± 0.1) (Table 3). Furthermore, four isolates of A. baumannii, four isolates of K. pneumoniae, and two isolates of P. aeruginosa were selected for time-kill analysis. We observed that rapid killing of these isolates occurred at 4 h of the assay. MP-B and the indolicidin combination had the synergistic activity against A. baumannii, K. pneumoniae, and P. aeruginosa isolates (FIC = 0.5 ± 0.2). The data presented in Table 3 showed two isolates of A. baumannii, three isolates of K. pneumoniae, and four isolates of P. aeruginosa exhibited FIC ≤ 0.37 (three fold decreases in log10 CFU/mL), however, nanoparticles exerted an additive effect within the assay time of 4 h incubation (Figure 1). The nano-TiO2 and nano-ZnO combination was more effective against K. pneumoniae as compared to other isolates, however, little bactericidal activity against A. baumannii. Furthermore, MP-B and indolicidin combination had biocidal activities on all bacterial isolates tested and drastically reduced viable counts of above species at 4 and 7 h of incubations (Figure. 1).
| Nanoparticles and Peptides | Number of Isolate | FICs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.18 | 0.25 | 0.31 | 0.37 | 0.5 | 0.53 | 0.56 | 0.62 | 0.75 | 1.0 | ||
| A. baumannii TiO2+ZnO | 7 | - | - | 1 | 3 | 3 | 1 | 3 | 5 | 3 | 1 |
| K. pneumonia TiO2+ZnO | 5 | - | - | 1 | 2 | 2 | 2 | 3 | 6 | 4 | - |
| A. baumannii MP + Indolicidin | 3 | - | - | - | 2 | 1 | - | - | 5 | 6 | 1 |
| K. pneumoniae MP + Indolicidin | 6 | - | - | - | 3 | 3 | - | 1 | 3 | 4 | 1 |
| P. aeruginosa MP + Indolicidin | 12 | 2 | 2 | 4 | 1 | 3 | 4 | 2 | 4 | - | 1 |
Abbreviations: FICs, Fractional inhibitory concentration indexes; MP, Mastoparan-B; Indo, Indolicidin.
aThe above values are in mg/mL. M9 containing glucose as source of carbon was used for nanometals and LB broth was used for peptides. 0.5 ≤ synergism, 0.5-1 = additive, 1- 4 = indifference, 4 ≥ antagonism. The results were repeated three times and similar observation was made. P ≤ 0.5 was considered statistically significant.
5. Discussion
With different types of antibiotic resistance genes and acquisition of plasmids spread, transposons and integrons among hospital isolates of A. baumannii, P. aeruginosa, and K. pneumoniae, as well as ever increasing failure in treatment of patients due to lack of proper treatment options, we decided to evaluate the susceptibility and synergistic effect of MP-B, indolicidin, nano-TiO2, and nano-ZnO as substitutes to antibiotics. Mastoparan-B peptide was the most potent biocide effective against A. baumannii, P. aeruginosa, and K. pneumoniae isolates (average MIC 4 mg/L), while indolicidin exerted bactericidal activity at higher MIC concentrations (MIC 16 mg/L), respectively. Furthermore, our findings suggest that the high MIC values of Tio2 and ZnO NPs may be related to a decrease in porosity of Gram- negative cell wall or existence of efflux pump that causes a decrease in intracellular concentrations of these compounds resulted in drastic reduction on biocidal activity of these compounds, even though we used synthetic medium to avoid precipitation of nanometals in the medium. The results were further supported by using combination of TiO2 and ZnO NPs that showed an additive effect on most of the A. baumannii and K. pneumoniae isolates (two fold decreases in log10 CFU/mL) but did not inhibit the growth of P. aeruginosa. However, synergism was observed when mastoparan-B and indolicidin combination were used at sub-MIC values by checker board titration tests. This is very interesting since the majority of burn infections caused by these microorganisms are resistant to carbapenem and make treatment very difficult.
It has been shown that the addition of ZnO NPs and TiO2 NPs generate reactive oxygen, causing an oxidative stress and bacterial cell death (25). Similarly, Khan et al., (25) reported that, ZnO and TiO2 NPs inhibited the growth and biofilm formation of the Streptococcus mitis ATCC 6249 at very low concentration. They suggested that ZnO and TiO2 NPs can be used as alternative antimicrobial agents for oral hygiene. Furthermore, recent investigation suggested that Campylobacter jejuni, the most common foodborne pathogen, was extremely sensitive to ZnO nanoparticles and the MIC for C. jejuni was determined to be 0.05 to 0.025 mg/mL, which was found to be 8 to 16 fold lower than that for Salmonella enterica Enteritidis and E. coli O157:H7 (0.4 mg/mL) (26).
The synergistic effect of the MP-B and indolicidin combination was further observed by time-kill assay. The results revealed that, at the sub-MIC concentrations, MP-B and indolicidin combination rapidly killed antibiotic resistant isolates, especially P. aeruginosa (reduced three-fold in log10 CFU/mL of the isolates), whereas, TiO2 + ZnO nanoparticles had practically no effect on these isolates alone, however, it virtually exerted an additive effect within 4 h incubation against certain isolates of A. baumannii and K. pneumoniae hospital isolates when used in combination but not P. aeruginosa strains. This finding is consistent with previous data (27) and demonstrates observed bactericidal effect against both Gram-positive and Gram-negative MDR bacteria over a 7 h incubation period when treated with sub-inhibitory concentrations of oregano essential oil and Ag NPs. Similar to our experiments, Vila-Farres et al., (28) found that, among 15 antimicrobial peptides tested, indolicidin and mastoparan showed good activity against both colistin-susceptible and colistin-resistant A. baumannii.
5.1. Conclusion
Our results suggest that antibacterial peptides MP-B and indolicidin in combination had a synergistic effect and may be a suitable candidate for antibiotics in treatment of topical infections associated with above bacteria.