1. Background
Pseudomonas aeruginosa is a ubiquitous microorganism which is present in diverse environmental niches and is seldom a member of normal human microbiota community (1). The capability of P. aeruginosa to tolerate different environmental conditions and to survive on minimal nutritional needs has allowed this opportunistic bacterium to survive in both general population and hospital units (2).
During patient hospitalization, colonization rates of this organism may exceed 50%, mainly among individuals who have experienced fracture or trauma in the skin or mucosal barriers due to surgical interventions, indwelling catheters, ventilation systems, tracheostomy, or burns (1, 3). Serious infections with P. aeruginosa are predominantly hospital-acquired. P. aeruginosa is principally problematic for critically ill patients in intensive care units (ICUs) and burn victims (4). This pathogen is a common causative agent of pneumonia (nosocomial pneumonia, ventilator-associated pneumonia, and healthcare-associated pneumonia), bacteremia, and urinary tract, skin, and soft tissue infections (5).
The virulence of P. aeruginosa is associated with the secretion of several cell-associated and extracellular factors. P. aeruginosa possesses a large number of virulence factors, such as exotoxin A, exoenzyme U, exoenzyme S, elastase, neuraminidase, phospholipase C, sialidase, and alkaline protease and exhibits antibiotic resistance. The virulence gene known as nan1 encodes a neuraminidase enzyme, which is responsible for attachment to the host’s respiratory tract.
Neuraminidase is an extracellular factor, known to play a vital role in the implantation of P. aeruginosa (6, 7). Class I integrons (encoded by int1 gene) possess two conserved segments (CS), separated by a variable region including integrated cassettes, which often consist of different antibiotic-resistant genes (7). P. aeruginosa is an increasingly problematic drug-resistant bacterium in today’s world. In fact, we are now faced with growing clones of pandrug-resistant P. aeruginosa in hospital settings, which threaten to move humans into what some consider the “post-antibiotic era” of bacterial diseases, caused by drug-resistant bacteria (8).
P. aeruginosa is a serious therapeutic problem for the treatment of both hospital- (nosocomial) and community-acquired infections; unfortunately, selection of the most effective drug is complicated, considering the capability of this bacterium to show resistance to several classes of antibiotics (9). This opportunistic pathogen owes its intrinsic and acquired resistance (to antimicrobial agents) mostly to the expression of chromosomally encoded efflux pumps, belonging to the so-called resistance-nodulation-cell division (RND) family of multidrug transporters.
P. aeruginosa can gain resistance to other antibacterial compounds either through the acquisition of antibiotic-resistant genes on mobile genetic elements (eg, transposon and plasmid) or by mutational mechanisms which change the expression and/or function of chromosomally encoded mechanisms (10). Not only is the rate of resistance to individual antibiotics or antibiotic classes a concern, but also emergence of multidrug-resistant isolates (resistance to at least three unrelated antibiotic classes) is an even more serious therapeutic problem (11).
Carbapenems (eg, imipenem and meropenem) have a broad spectrum of anti-bacterial activities and are increasingly used as the last-resort antibiotic treatment of serious infections, caused by multi-drug resistant P. aeruginosa isolates (12). Epidemiological research has revealed that diseases caused by drug-resistant P. aeruginosa are associated with a significant increase in the length of hospital stay, intensive care, morbidity, mortality, need for surgical interventions, and the overall cost of treating patients and families (13). Also, antibiotic resistance can emerge during the course of therapy.
Selection of the most appropriate drug to initiate treatment is vital to improving the clinical results. Given the changes in the antibiotic resistance profile of isolates in each region and hospital setting, it is important for each area to formulate its antibiotic stewardship according to the regional resistance patterns. Therefore, in this study, we aimed to study the antibiotic resistance and virulence factors of P. aeruginosa.
2. Objectives
The aim of this study was to examine the antibiotic resistance patterns and presence of nan1 and int1 virulence genes (encoding neuraminidase and class 1 integrons, respectively) in P. aeruginosa clinical isolates and to analyze the values with respect to the hospital wards, specimens, and antibiotic resistance of the strains.
3. Methods
3.1. Isolation and Confirmation of P. aeruginosa Isolates
In this cross sectional study, strains recovered consecutively from different samples (blood, tracheal tube, urine, wound, and sputum) of hospitalized patients in different wards between 2014 and 2016 in Gonabad, Iran, were tested. Briefly, aerobic blood culture was performed, using a manual blood cultivator tube. Aerobic culture of other specimens was performed on common bacteriological culture media, including blood agar (BA), chocolate agar, and MacConkey (MC) agar (Merck Co., Germany). All cultures were maintained under incubation at 35 ± 2°C for 24 hours.
Characterization and identification of bacteria were carried out, based on standard procedures. Bacterial colonies were removed and Gram staining and subcultures were performed on MC agar. The isolates were recognized as P. aeruginosa, based on Gram staining, colony morphology, oxidase test, motility, catalase test, pigment production, triple-sugar iron fermentation, H2S production, urease level, indole test, and citrate utilization test.
For the final confirmation of the isolates, different biochemical tests, embedded in the API-20NE diagnostic system (bioMerieux, France), were applied (4). The isolates identified as presumptive P. aeruginosa were further specified at species level by species-specific polymerase chain reaction (PCR). The isolated strains were stored in brain-heart infusion (BHI) broth (Merck Co., Germany), containing 20% (v/v) glycerol (Merck Co., Germany) at -20°C until the antimicrobial susceptibility test was conducted in the laboratory of clinical microbiology.
3.2. Antibiotic Susceptibility
P. aeruginosa strains were cultured from BHI broth-glycerol stocks on MC medium and were grown overnight at 35 ± 2°C. All the isolates were tested for their antimicrobial susceptibility patterns, using the standard guidelines issued by the clinical and laboratory standards institute (CLSI) (14). All the inoculated plates were incubated for 16-18 hours in an ambient air incubator at 35 ± 2°C, and the results were recorded by measuring the inhibition zone as sensitive (S), intermediate (I), or resistant (R), according to the CLSI guidelines.
Susceptibility to 24 antimicrobial agents (MAST Co., UK) was tested with disc diffusion method on cation-adjusted Mueller-Hinton Agar plates, according to the CLSI recommendations (15). The agents included ciprofloxacin (CIP, 5 μg), cefepime (CPM, 30 μg), doripenem (DOR, 10 μg), norfloxacin (NOR, 5 μg), ofloxacin (OFX, 5 μg), gentamicin (GM, 10 μg), colistin (CO, 10 μg), ceftazidime (CAZ, 30 μg), imipenem (IMP, 10 μg), piperacillin-tazobactam (PTZ, 100/10 μg), piperacillin (PRL, 100 μg), tobramycin (TB, 10 μg), aztreonam (ATM, 30 μg), ticarcillin (TC, 75 μg), amikacin (AK, 30 μg), meropenem (MEM, 10 μg), ticarcillin-clavulanate (TIM, 75/10 μg), and levofloxacin (LEV, 5 μg). For the quality control, P. aeruginosa ATCC 27853 was used.
3.3. PCR Assay
Bacterial DNA extraction: A single pure colony of each isolate was inoculated from the MC agar plate into 5 mL of Mueller-Hinton broth and incubated for 16 to 18 hours at 35 ± 2°C. Subsequently, 1.5 ml of the overnight culture was harvested by centrifugation at 7000 g for 5 minutes. After the supernatant was decanted, the pellet was resuspended in 500 μL of deionized water. The bacterial cells were lysed by heating at 95°C for 10 minutes. Then, bacterial cell debris was removed by centrifugation at 13000 g for 5 minutes. The supernatant of the total sample was applied as the main source of DNA template for PCR reaction (16).
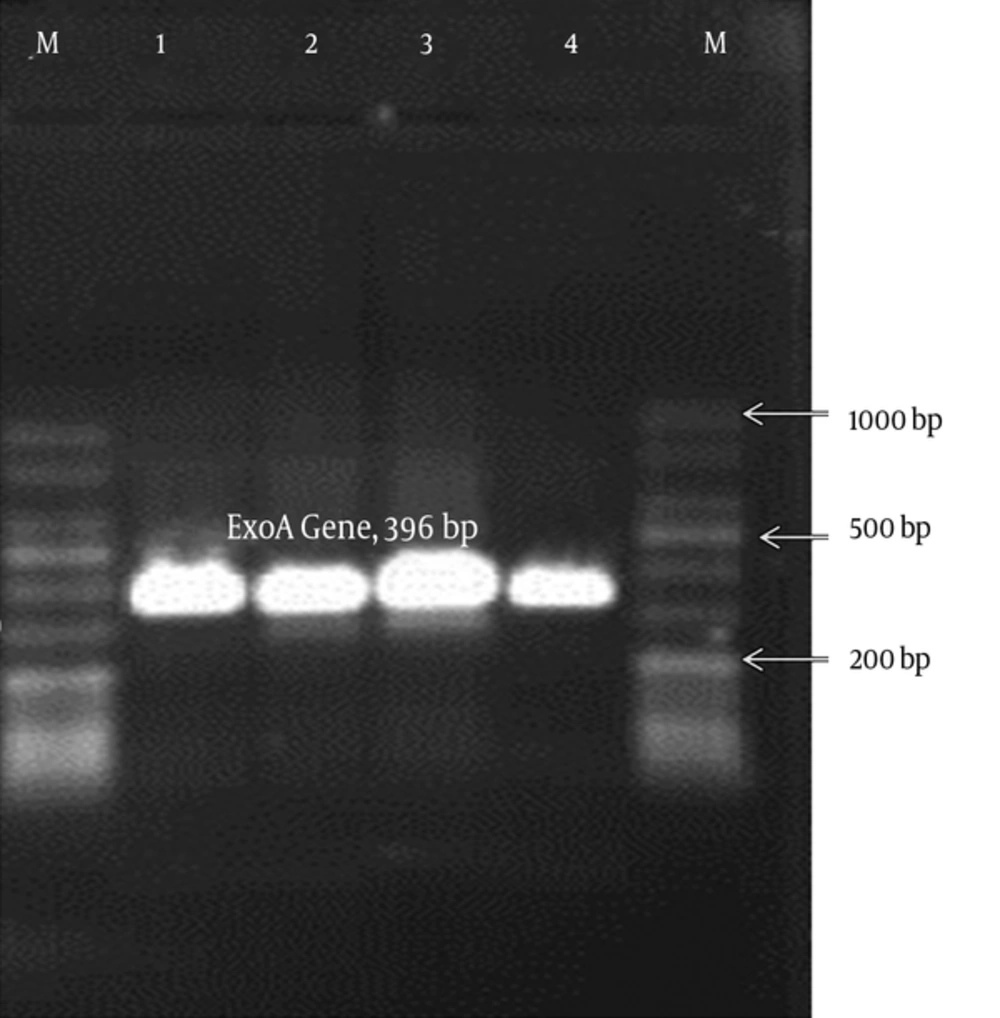
Molecular confirmation of the isolates as P. aeruginosa: For molecular confirmation of the isolates confirmed by conventional tests, PCR was performed. All the isolates, identified as presumptive P. aeruginosa by morphological and biochemical tests, were further confirmed by species-specific PCR to detect the exoA gene. The exoA primer sequences, annealing temperature, and the expected size of amplicon for PCR assay are presented in Table 1. P. aeruginosa ATCC 27853 was used as the positive control.
| Virulence Factor | Target Gene | Primer Name | Sequence (5’ to 3’) | Length (Base) | Annealing | Amplicon Size, pb | References |
|---|---|---|---|---|---|---|---|
| Exotoxin A | ExoA | ExoA-F | GACAACGCCCTCAGCATCACCAGC | 24 | 68 | 396 | (15) |
| ExoA-R | CGCTGGCCCATTCGCTCCAGCGCT | 24 | |||||
| Class 1 integrons | Int1 | Int1-F | GTTCGGTCAAGGTTCTG | 17 | 50 | 923 | (17) |
| Int1-R | GCCAACTTTCAGCACATG | 18 | |||||
| Neuraminidase | nan1 | nan1-F | ACGCTCCGTCCAGCCGGA | 18 | 60 | 221 | (18) |
| nan1-F | GTCTGGACGACGGCGGCA | 18 |
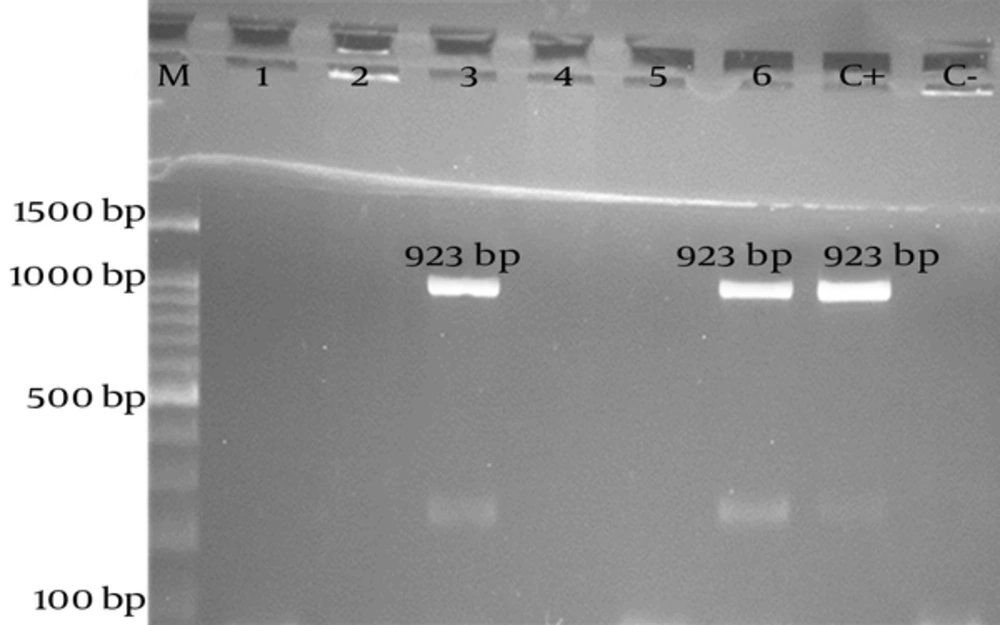
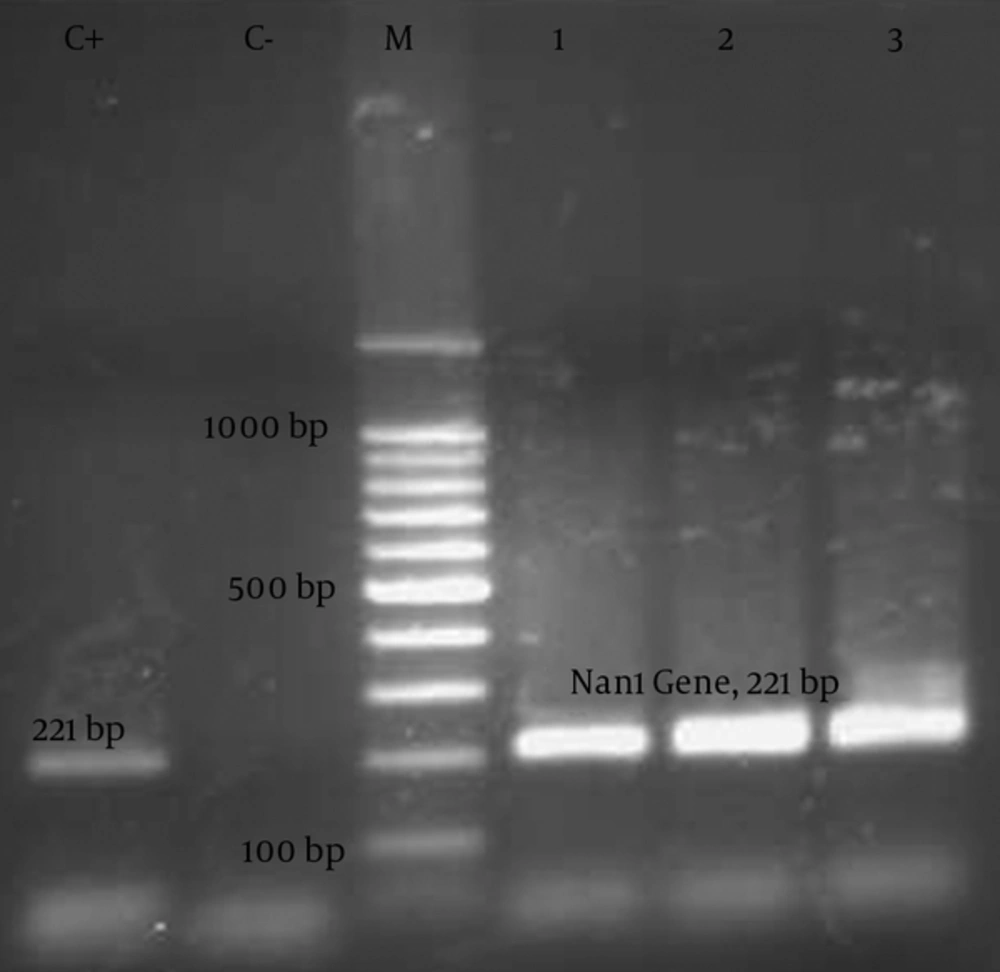
Detection of virulence factors and antibiotic resistance markers in P. aeruginosa isolates: Genes encoding the virulence factors (nan1 and int1) were investigated by PCR, using specific primers. The PCR assays have been previously described by other researchers (Table 1). The primer sequences, annealing temperature, and expected size of amplicons for each PCR assay are shown in Table 1 (15, 17, 18). PCR reaction was carried out with 1X PCR buffer, 2 mM of MgCl2, 0.2 mM of deoxyribonucleoside triphosphates (dATP, dCTP, dGTP, and dTTP), 0.25 µM of each primer (forward and reverse primers), 1.5 U of Taq DNA polymerase (Jena Bioscience, Germany), and 3 µL of template DNA in a total volume of 25 µL.
PCR reaction was performed and the amplicons were analyzed by electrophoresis on 1% agarose gel, using tris-borate-EDTA buffer (pH = 8.0). Subsequently, the agarose gel was stained with ethidium bromide and visualized on an ultraviolet transilluminator system. The expected size of amplifications (PCR products) was estimated by comparison with a related DNA ladder (Figures 1 - 3).
3.4. Statistical Analysis
Statistical analysis of the results was performed by Pearson’s Chi-square test, and the significance level was set at P < 0.05, using SPSS version 22.
4. Results
4.1. Patient Data
A total of 95 P. aeruginosa isolates were studied during the study period. The isolates were recovered from 30 (31.6%) males and 65 (68.4%) females. Overall, 34 (35.5%) infected patients were included in the age group of 30 - 44 years. There were 24 (25.3%) patients hospitalized in the intensive care unit (ICU). A total of 31 (32.6%) strains were isolated from blood; therefore, blood was the most common specimen from which the isolates were recovered. Table 2 shows the demographic features of the patients infected with P. aeruginosa.
| Categories | Resultsa |
|---|---|
| Gender | |
| Male | 30 (31.6) |
| Female | 65 (68.4) |
| Age, y | |
| < 30 | 10 (10.5) |
| 30 - 44 | 34 (35.5) |
| 45 - 59 | 30 (31.6) |
| > 60 | 21 (22.1) |
| Ward | |
| Burn | 23 (24.2) |
| Internal medicine | 23 (24.2) |
| ICU | 24 (25.3) |
| CCU | 5 (5.3) |
| Surgery | 14 (14.7) |
| Women | 6 (6.3) |
| Specimen | |
| Wound | 18 (18.9) |
| Sputum | 7 (7.4) |
| Blood | 31 (32.6) |
| Tracheal tube | 12 (12.6) |
| Urine | 27 (28.4) |
aValues are expressed as No. (%).
4.2. Phenotypic Data and Antibiotic Susceptibility Patterns
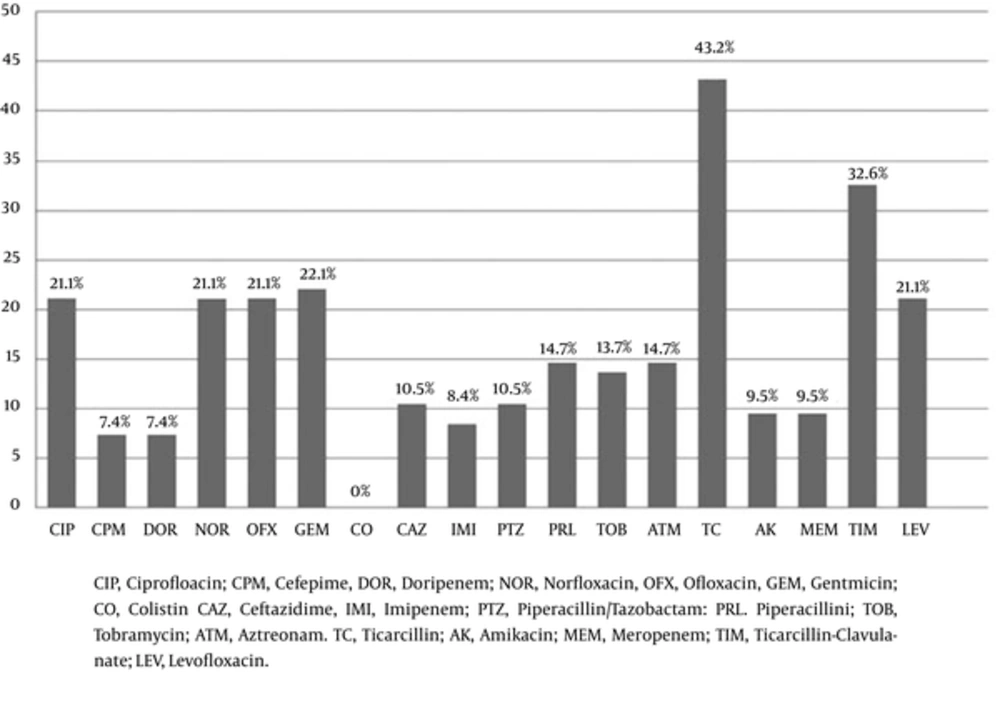
As observed on Mueller-Hinton Agar plate, the green and yellow pigments were seen in 75.8% and 20% of the isolates, respectively. According to the in vitro antibiotic susceptibility test, colistin was the most effective antibiotic against the isolates (100% of the isolates were sensitive), while ticarcillin was the least effective antimicrobial agent (43.2% of the isolates were resistant). As revealed, 21.1% of P. aeruginosa strains were resistant to the quinolone class of antimicrobial agents (ciprofloxacin, ofloxacin, norfloxacin, and levofloxacin).
Also, ceftazidime resistance was detected in the isolates (10.5%). Resistance rates for the studied isolates are presented in Figure 1. In the current study, 7.4% of the isolates were resistant to fourth-generation cephalosporins (cefepime). Characteristics of cefepime-resistant P. aeruginosa isolates are shown in Table 3. All these 7 isolates were resistant to ceftazidime and imipenem, and five of them had nan1 virulence factor.
| Isolate No. | Specimen | Hospital Ward | Pigment Color | Virulence Factor | Antimicrobial Susceptibility Pattern | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nan1 | Int1 | CO | CAZ | IMI | AK | CIP | PTZ | ||||
| 3 | Wound | ICU | Green | - | - | S | R | R | R | S | R |
| 4 | Wound | Burn | NP | - | - | S | R | R | R | R | S |
| 23 | Tracheal tube | ICU | Green | + | - | S | R | R | R | R | R |
| 34 | Urine | Internal | Green | + | + | S | R | S | S | R | S |
| 53 | Blood | Internal | Yellow | + | - | S | R | R | R | R | R |
| 58 | Wound | Burn | Yellow | + | + | S | R | R | R | R | R |
| 92 | Tracheal tube | ICU | Yellow | + | - | S | R | R | R | R | R |
Abbreviations: Ak, amikacin; CAZ, ceftazidime; CIP, ciprofloxacin; CO, colistin; ICU, intensive care unit; IMI, imipenem; NP, non-pigmented; PTZ, piperacillin/tazobactam; R, resistant; S, sensitive.
As multiple antibiotic resistance results were interpreted, about 5.26% of the tested isolates were co-resistant to ceftazidime, amikacin, and piperacillin/tazobactam. Table 4 depicts the multiple antibiotic resistance patterns of the isolates. The most common patterns were TIMR-TCR (31 isolates), followed by TIMR-PTZR (10 isolates) and GEM, TOB, AK (7 isolates).
| Pattern | Antibiotic Resistance Patterns | No. (%) |
|---|---|---|
| A | IMI, MEM, CPM, CAZ | 6 (6.31) |
| B | MEM, NOR, CAZ, TOB | 5 (5.26) |
| C | CIP, CPM, GEM | 6 (6.31) |
| D | CIP, CAZ, TOB | 6 (6.31) |
| E | CAZ, AK, PTZ | 5 (5.26) |
| F | GEM, TOB, AK | 7 (7.36) |
| G | IMI, MEM, DOR | 6 (6.31) |
| H | TIM, PTZ | 10 (10.52) |
| I | TIM, TC | 31 (32.63) |
| J | PRL, AK, CAZ | 5 (5.26) |
| K | AK, TOB, GEM | 5 (5.26) |
Abbreviations: AK, amikacin; ATM, aztreonam; CAZ, ceftazidime; CIP, ciprofloxacin; CO, colistin; CPM, cefepime; DOR, doripenem; GEM, gentamicin; IMI, imipenem; LEV, levofloxacin; MEM, meropenem; NOR, norfloxacin; OFX, ofloxacin; PRL, piperacillin; PTZ, piperacillin/tazobactam; TC, ticarcillin; TIM, ticarcillin-clavulanate; TOB, tobramycin.
4.3. Detection of Virulence-Associated Genes
All bacterial isolates, identified as presumptive P. aeruginosa by diagnostic microbiological (morphological and biochemical) tests, were further confirmed by species-specific PCR to detect the exoA gene. Gene exoA was detected in all 95 isolates. Among 95 P. aeruginosa isolates on which PCR assay was performed, approximately 44.2% had nan1 gene. Isolates harboring Int1 gene were only detected in 29 isolates (30.5% of the strains) (Figure 4).
5. Discussion
Treatment of diseases caused by P. aeruginosa has become increasingly difficult. The consecutive increase in the population of immunocompromised patients and the evolutionary features of pathogenic bacteria to promptly mutate and adapt to antibacterial threats in the environment (eg, hospital) make the treatment of bacterial infections a serious problem (1).
This study investigated the demographic and microbiological features of patients infected with P. aeruginosa. In our study, consistent with previous research, the majority of the studied patients were female and older than 30 years (3). This may be due to factors such as differences in the immune system, lifestyle, and professional performance of individuals infected with this opportunistic pathogen. In the present study, patients hospitalized in the ICU were the main susceptible group to P. aeruginosa infections, which is also noted in other studies (19).
The percentage of carbapenem-resistant isolates in the present research is comparable to other studies in Iran (4). Evidently, the prevalence rates vary in different studies, which could be explained by differences in the sample size, distribution of risk factors, and treatment regimen patterns. In the present study, 8.4% of the isolates were imipenem-resistant, similar to previous research (4). Nevertheless, Franco and colleagues reported that 34.5% of blood isolated strains were resistant to imipenem in 2010 (20).
In the current study, 7.36% of the bacteria were resistant to the aminoglycoside antibiotic class (9.5% of the isolates were resistant to amikacin). Raja et al. reported that 6.73% of P. aeruginosa isolates were resistant to amikacin in Malaysia (21), which is in agreement with the present findings. In a multicenter study in Iran, the results showed that 20% of the isolates were resistant to amikacin (4). In another study in Pakistan on P. aeruginosa strains isolated from lower respiratory tract infections, Fatima and colleagues showed that 35% of the isolates were resistant to amikacin (22). Our results showed that 22.1% of the strains were resistant to gentamicin.
The antibiotic resistant patterns reported by Jafari and colleagues showed that 49% of the isolates were resistant to gentamacin (23). Various studies have shown different resistance patterns, which may be due to differences in treatment regimens used for infected patients in healthcare settings. To alleviate the rate of multi-drug resistance (MDR) and spread of resistance, clinicians should switch to therapies with a narrower spectrum. In the current study, 6.31% of the isolates had an IMIR-MEMR-CPMR-CAZR pattern; therefore, these drugs are not suitable for the treatment of P. aeruginosa-related infections. Our findings also showed that all the bacterial strains (100%) were susceptible to colistin. However, the point to be noted is that colistin is a toxic antibiotic and used as a last-line treatment option for the treatment of infections caused by P. aeruginosa.
The frequency of nan1 gene among the tested P. aeruginosa was high (44.2%), which suggests the key role of neuraminidase enzyme in the pathogenesis of this group of isolates. The propagation of virulence-related genes varied with respect to the site of infection in infected patients. In this regard, Mitov et al. reported 15.4% distribution of nan1 gene in wound isolates from patients and a variable frequency rate of the gene in the isolates from different specimens of affected patients (6.7% to 62.5%) (18). In the present study, among 7 cefepime-resistant P. aeruginosa isolates, 5 had the nan1 gene. In our study, the frequency of int1 gene (30.5%) was lower than those reported by Gu et al. in China (40.8%) (24). This may be due to differences in the distribution of antibiotic resistance in various geographical regions.
The point to be noted is that P. aeruginosa is intrinsically resistant to penicillin (ie, benzylpenicillin), first-generation cephalosporin (cephalothin and cefazolin), second-generation cephalosporin (cefuroxime), cephamycin (cefoxitin and cefotetan), ampicillin, amoxicillin, ampicillin-sulbactam, amoxicillin-clavulanate, cefotaxime, ceftriaxone, ertapenem, tetracycline, tigecycline, trimethoprim, trimethoprim-sulfamethoxazole, chloramphenicol, and fosfomycin. Therefore, these drugs are not clinically effective and should not be reported. In fact, intrinsic antibiotic resistance is so common that in vitro susceptibility testing is unnecessary (14).
Today, a major concern of medical and clinical practitioners is the emerging MDR and pandrug-resistant pathogenic bacteria and their associated complications in developing countries (25-28). This is principally true for P. aeruginosa and its capability to emerge as MDR clones. The most difficult challenge of P. aeruginosa infections is its capability to promptly show resistance to several unrelated classes of antibiotics during the period of clinical therapy.
The emerging outbreak of MDR and PDR P. aeruginosa strains is related to different factors, such as its inherent resistance to a variety of antibiotics, its capability to gain antimicrobial resistance determinants, history of surgical interventions and chronic infections, irrational use of antibiotics, and abundance use of broad-spectrum antibiotics, which increase the selection of bacterial resistant clones (27).
Synergistic responses are an important feature of some drug combinations (eg, piperacillin-tazobactam). The main focus of combination-antibiotic therapy against P. aeruginosa is preventing the emergence of antimicrobial resistance and treatment failure. The combination of an anti-Pseudomonal-beta-lactam with an aminoglycoside has been often the therapeutic regimen of choice for this pathogenic bacterium. In this regard, antibiotic resistance trends from large multicenter and national surveillance studies provide important information. It should be also noted that in many cases, antibiotic resistance is transmitted to the human community and healthcare settings through other environmental and food sources (29-35).
Simultaneous determination of antibiotic susceptibility profiles and virulence determinants is a contemporary approach for the examination of microbiological aspects of infections caused by P. aeruginosa. The large sample size of the present study allowed elucidation of statistically significant results with regard to the prevalence of antibiotic resistance and virulence genes in the tested isolates from markedly varying sites and sources of isolation.
Finally, selection of the most effective anti-Pseudomonal drug (including in vitro test and report) is a decision best made by each clinical microbiology laboratory in consultation with the infectious diseases practitioners and pharmacologists, as well as therapeutic and hospital infection control committees.