1. Background
Acute kidney injury (AKI) is a frequently encountered problem among ill neonates, admitted to neonatal intensive care units (8% - 24%) (1, 2). Decreased renal perfusion, low glomerular filtration rate, increased renal vascular resistance, and high plasma renin activity are major predisposing factors for acute renal impairment in the first days of life (3, 4).
Neonatal AKI is usually transient and reversible with appropriate treatment of underlying disorders (5). Some known contributing factors for AKI include: neonatal septicemia, respiratory distress syndrome, low birth weight, prematurity, cesarean section, intracranial hemorrhage, perinatal asphyxia, intubation at birth, preeclampsia, premature rupture of membranes, male gender, surgical procedures, mechanical ventilation with high positive airway pressure, dehydration, genitourinary abnormality, necrotizing enterocolitis, drug nephrotoxicity, and antenatal steroid treatment.
Generally, more than one contributing factor is involved in the pathogenesis of AKI in neonates with septicemia (6-10). A few original studies have been performed concerning AKI in neonates with septicemia. The aim of this study was to identify the risk factors and clinical course of AKI in neonates with documented septicemia.
2. Methods
This study was performed on 138 outborn neonates with culture-proven septicemia in 2 groups with (n, 65) and without (n, 73) AKI during 2005 - 2016. Septicemia was suspected in neonates with fever, poor feeding, vomiting, lethargy, or irritability; it was confirmed by a positive blood culture. Blood culture was performed under sterile conditions after skin disinfection. On the other hand, neonates with severe perinatal asphyxia, maternal history of renal dysfunction, major congenital anomalies, congenital anomalies of the kidney and urinary tract, genetic syndromes, and postoperative AKI were excluded.
Neonatal septicemia was classified into early and late onset, ie, development of septicemia in the first 3 days or after 3 days of life, respectively. Diagnosis of AKI was established based on one or more of the following criteria: serum creatinine (Cr) > 1.5 mg/dL after the first 48 - 72 hours of life with normal maternal serum Cr, doubled serum Cr level during hospital admission or increasing serum Cr level at a rate of 0.3 mg/dL/24h.
The present study was approved by the Institutional Review Board, and verbal consent was obtained from the legal parents of all newborns before enrollment. The primary outcomes included death or survival with or without renal impairment. Medical records were reviewed for demographic characteristics and laboratory results, including gestational age, birth weight, age at admission, age of renal failure, gender, Apgar score at 1 and 5 minutes, blood pressure, mode of delivery, premature rupture of membranes, associated infections, intraventricular hemorrhage, mechanical ventilation, convulsion, feeding problems, umbilical catheterization, urine output, serum electrolyte level, and pH at admission.
Renal function tests (blood urea nitrogen [BUN] and Cr) were performed at admission, 72 hours post admission, during the first week, and at discharge. Serum Cr concentration was ignored in the first 2 days of life, as it reflected the maternal value. The values of the study variables were defined as follows:
- Low birth weight: birth weight, 1500 - 2500 g; very low birth weight: birth weight, 1000 - 1500 g
- Prematurity: birth at < 37 weeks of gestation, as confirmed by antenatal ultrasound and Ballard postnatal scoring
- Premature rupture of membranes: rupture of membranes for more than 18 hours before the onset of labor
- Hypothermia: body temperature < 36.5°C and fever (body temperature > 37.5°C)
- Leukopenia: white blood cell (WBC) count < 5000/mm3; leukocytosis: WBC count > 30000/mm3 in the first day of life, followed by> 15000 - 20000/mm3
- Thrombocytopenia: platelet count < 150000/mm3; thrombocytosis: platelet count > 400000/mm3
- Anemia: hemoglobin level < 14 g/dL in term infants and 12 - 13 g/dL in very-low-birth-weight infants
- Metabolic acidosis: serum pH < 7.20 in the first day of life and < 7.35 afterwards
- Hyponatremia: serum Na < 133 mmol/L; hypernatremia: serum Na > 150 mmol/L
- Hypokalemia: serum potassium < 3.2 mmol/L in the first week of life and < 3.4 mmol/L at 1 week to 1 month; hyperkalemia: serum potassium > 5.5 mmol/L in the first week and > 6 mmol/L at 1 week to 1 month of life
- Hypocalcemia: serum calcium < 9 mg/dL on the first day, < 7 mg/dL during 1 - 2 days, and < 9 mg/dL in 4 - 7 days of life
- Azotemia: serum BUN > 25 mg/dL in premature infants and > 12 mg/dL in term infants
- Hypertension: systolic and/or diastolic blood pressure > 95% for gestational age
- Oliguria: urine volume < 1 mL/kg/hour after 48 hours of birth
Neonates with early-onset sepsis were treated with ampicillin and aminoglycosides, while those with late-onset septicemia received vancomycin and aminoglycosides. Neonates who appeared to have prerenal dysfunction (oliguria, BUN/Cr > 20, and hypovolemia) were subjected to fluid challenge (20 cc/kg/NS, DW5%) until euvolemic; if oliguria persisted, 2 mg/kg of lasix infusion was used. Intrinsic renal failure was suggested in patients with intractable oliguria, unresponsive to fluid challenge.
2.1. Statistical Analysis
Statistical analysis was performed using SPSS version 22 (Chicago, IL, USA). Normal distribution of continuous variables was assessed by Kolmogorov-Smirnov test. Normally distributed continuous variables were assessed by independent sample t test, whereas Mann-Whitney U test was used for the comparison of continuous variables without a normal distribution. Chi square test was used to evaluate qualitative binary data, and Fisher’s exact test was applied to 2 × 2 contingency tables with at least 1 expected cell less than 5.
All variables, which might predict AKI, were assessed by univariate analysis, and variables with P value ≤ 0.2 were included in the multivariable analysis. Crude and adjusted odds ratios (ORs) were obtained by stepwise backward logistic regression. Removal probability less than 0.1 was considered for the stepwise analysis. Moreover, the receiver operating curve (ROC) analysis was used to determine the optimal cutoff point of sensitivity and specificity versus the gold standard by STATA SE version 11.
3. Results
A total of 65 patients with AKI (male, 61.5%) and 73 patients without AKI (male, 63%) were enrolled in this study. The mean age of patients at diagnosis of AKI was 11.31 ± 9.92 days. Renal failure was nonoliguric in the majority of patients (77.4%), and septicemia was the only cause of AKI in 40% of patients. However, 60% of patients had additional predisposing factors, such as associated infections, multidrug treatment, associated disorders, and mild birth asphyxia. Ultrasound was normal in 84.2% of cases, while it showed increased echogenicity in 10.5% of patients. In total, 15% of patients died and 85% were discharged without residual renal impairment. None of the patients needed renal replacement therapy.
There was no significant difference in terms of gender, onset of sepsis, WBC count, hemoglobin level, and serum electrolytes between the groups. However, in neonates with AKI, significantly lower age at admission, gestational age, birth weight, blood pressure, platelet count, and serum pH at admission were reported. AKI patients also showed a higher incidence of intubation at birth, inotrope treatment, mechanical ventilation, shock, DIC, and mortality, compared to the control group.
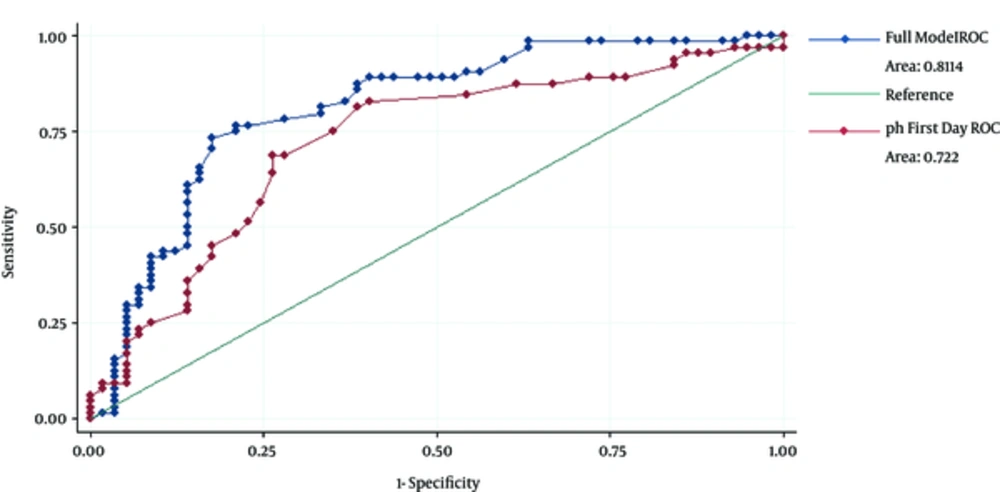
In addition, gestational age, DIC, and serum pH were the only independent predictors of AKI in the multiple backward stepwise regression analysis. Tables 1 - 4 show the significance of demographic and laboratory findings in the groups. The area under curve (AUC) for serum pH at admission was 0.72 (CI, 0.63 - 0.81), which is suggestive of a relatively accurate factor for the prediction of AKI (P = 0.015) (Figure 1). With an optimal cutoff point of 0.46, the sensitivity and specificity of initial pH were 83% and 60%, respectively (Table 5).
| Variables | Normal (%) | High (%) | Low (%) |
|---|---|---|---|
| Gestational age (w) | 23.1 | - | 76.9 |
| Birth weight (g) | 24.6 | - | 75.4 |
| Blood pressure | 88.5 | - | 11.5 |
| WBC (mm3) | 68 | 18.4 | 13.6 |
| Hgb (g/dL) | 40 | - | 60 |
| Platelet (mm3) | 62.5 | 1.6 | 35.9 |
| Na (meq/L) | 77.4 | 3.2 | 19.4 |
| K (meq/L) | 78.5 | 1.5 | 20 |
| Ca (mg/dL) | 82.8 | 17.2 | - |
| Final Cr (mg/dL) | 70 | 30 | - |
| Serum pH | 39.1 | 6.3 | 54.6 |
| Variables | Case (n, 65) | Control (n, 73) | P Value |
|---|---|---|---|
| Gender (m) | 40 (61.5) | 46 (63) | NSb |
| Gestational age (t) | 15 (23) | 43 (60) | < 0.001b |
| Mean gestational age (w) | 33.5 (3.9) | 36.8 (3.7) | < 0.001c |
| Intubation at birth | 18 (28) | 7 (9.6) | 0.008b |
| Inotrope treatment | 42 (64.6) | 19 (26) | < 0.001b |
| Shock | 21 (32) | 5 (7) | < 0.001b |
| DIC | 23 (35.4) | 4 (5.5) | < 0.001b |
| Early-onset sepsis | 30 (46) | 25 (34) | NS |
| Mechanical ventilation | 31 (48) | 12 (16.4) | < 0.001b |
| Mortality | 10 (15.4) | 3 (4) | 0.038b |
| Admission age (d) | 8.3 (9) | 11.3 (9.9) | 0.039c |
| Birth weight (g) | 2005 (816) | 2669 (820) | < 0.001d |
| WBC (mm3) | 11203 (9365) | 9974 (5409) | NS |
| Hgb (g/dL) | 12.7 (2.7) | 12.4 (2.6) | NS |
| Platelet (mm3) | 198506 (122455) | 289094 (151259 | < 0.001d |
| CRP | 1.8 (1) | 1.5 (1) | 0.049c |
| Na (meq/L) | 136 (5.6) | 138 (5) | NSd |
| K (meq/L) | 4.9 (1) | 5 (1) | NS |
| Ca (mg/dL) | 8.7 (0.97) | 8.8 (1) | NS |
| Blood pressure | 57.6 (8.5) | 63 (11.5) | 0.003d |
| Serum pH | 7 (0.2) | 7.4 (0.12) | < 0.001c |
aValues are presented as mean (SD) or number (percentage).
bChi square test.
cMann-Whitney U test.
dIndependent sample t test.
| Variables | OR (95% CI) | Chi Square Results |
|---|---|---|
| Gender (m) | 0.9 (0.47, 1.9) | 0.8 |
| Gestational age (t) | 5.3 (2.5,11.2) | < 0.001 |
| Mean gestational age (w) | 0.8 (0.7,0.9) | < 0.001 |
| Intubation at birth | 0.28 (0.1, 0.7) | 0.008 |
| Inotrope treatment | 0.2 (0.09, 0.4) | < 0.001 |
| Shock | 0.15 (0.5, 0.4) | < 0.001 |
| DIC | 0.1 (0.03,0.3) | < 0.001 |
| Early-onset sepsis | 0.6 (0.3, 1.3) | 0.13 |
| Mechanical ventilation | 0.2 (0.1, 0.5) | < 0.001 |
| Age at admission (d) | 0.97 (0.9, 1.003) | 0.07 |
| Birth weight (g) | 0.99 (0.99, 0.99) | < 0.001 |
| WBC (mm3) | 1 | 0.34 |
| Hgb (g/dL) | 1.05 (0.93, 1.2) | 0.4 |
| Platelet (mm3) | 1 | 0.9 |
| CRP | 1.4 (0.96, 2) | 0.07 |
| Na (meq/L) | 0.96 (0.92, 1.02) | 0.2 |
| K (meq/L) | 0.9 (0.7, 1.3) | 0.7 |
| Ca (mg/dL) | 0.9 (0.6, 1.2) | 0.4 |
| Blood pressure | 0.94 (0.9, 0.98) | 0.005 |
| Serum pH | 0.008 (0.0004, 0.2) | 0.002 |
| Variables | Adjusted OR (95% CI) | P Value |
|---|---|---|
| Gestational age (t) | 3.5 (1.3, 9) | 0.01 |
| Intubation at birth | - | |
| Inotrope treatment | - | |
| Shock | - | |
| DIC | 0.16 (0.04, 0.63) | 0.009 |
| Mechanical ventilation | - | |
| Admission age (d) | - | |
| Birth weight (g) | - | |
| CRP | - | |
| Blood pressure | - | |
| Serum pH | 0.015 (0.001, 0.4) | 0.015 |
Abbreviations: d, day; g, gram; m, male; t, term; w, week.
| Variables | Sensitivity (CI) | Specificity (CI) | AUC (CI) | Cutoff | P Value |
|---|---|---|---|---|---|
| Complete model | 0.89 (0.79, 0.95) | 0.6 (46, 0.7) | 0.81 (0.73, 0.89) | 0.44 | - |
| pH | 0.83 (0.7, 0.89) | 0.6 (0.48, 0.72) | 0.72 (0.63, 0.81) | 0.46 | 0.015 |
| DIC | 0.36 (0.25, 0.47) | 0.95 (0.87, 0.98) | - | - | - |
| Gestational age | 0.77 (0.65, 0.85) | 0.62 (0.5, 0.72) | - | - | - |
4. Discussion
Renal dysfunction occurs in 15% - 20% of neonates with septicemia. Pathophysiological mechanisms of AKI include hypoxic-ischemic insults with tubular damage, transient pyelonephritis, vascular destruction, myoglobinuria, hemorrhage, and DIC with peritubular capillary obstruction (3, 11). Recovery and prognosis of acute renal failure depend on the underlying etiology, early diagnosis, hemodynamic instability, multiorgan failure with application of mechanical ventilation, vasopressor treatment, and renal replacement therapy (7, 9, 11).
Most patients in the present study had predisposing factors for AKI, such as localized infections, multiple drug administration, and mild birth asphyxia. However, renal function improved in the majority of patients by appropriate antibiotic therapy and supportive management, with no residual renal impairment, which is in accordance with previous reports (11, 12). Similar to previous studies, AKI occurred more commonly in male than female neonates in our study, which is secondary to the increased incidence of septicemia and respiratory distress syndrome in male infants (8-10). However, in a study by Momtaz et al., a higher incidence was reported in female neonates (13).
Consistent with previous studies (5, 9), AKI was more frequent in premature and low-birth-weight infants in our study, relative to the extent of prematurity. In addition, premature infants with severe septicemia, acidosis, and DIC had a higher incidence of renal failure. In a study by Mathur et al., low birth weight was an important risk factor for AKI in neonates with septicemia and was more common in small and sick infants. Intrauterine insults, fetal distress, maternal medications, congenital anomalies, low glomerular filtration rate, placental insufficiency, and infections are the main causes of AKI in premature infants. The postnatal course of premature infants is often complicated with septicemia, hypovolemia, hypotension, drug toxicity, mechanical ventilation, and renal ischemia (7).
Nonoliguric renal failure occurs more commonly in neonatal septicemia, with favorable prognosis for preserved fluid and electrolyte hemostasis (3, 10, 11). Meanwhile, oliguric renal failure may occur in 15-93% of neonates with severe and prolonged septicemia (3, 8, 9, 11). The majority of our patients had nonoliguric renal failure with no residual renal impairment.
Prerenal azotemia has been recognized as the most common cause of neonatal AKI in different studies (64% - 85%). However, intrinsic and postrenal failure have been reported in 11% - 52% and 3% of patients, respectively (5, 9, 14). Similarly, most of our patients had prerenal azotemia and were responsive to fluid challenge.
In spite of recent advances in renal replacement therapies, mortality of AKI remains high (25% - 78%) (2, 6). It occurs in 44% - 78% of patients with neonatal septicemia, and the risk is 2 - 3 times higher in these patients, compared to those without AKI (3, 14). In the present study, about 15% of patients died and 85% were discharged. Overall, the predisposing factors include underlying etiology, female gender, birth asphyxia, low birth weight, multiorgan failure, hypotension, septicemia, profound acidosis, shock, oligoanuria, nephrotoxic drugs, vasopressor treatment, mechanical ventilation, and peritoneal dialysis (13, 15).
In conclusion, it is recommended to monitor renal function tests in all neonates with septicemia (11). Early diagnosis and management of septicemia, in addition to abnormalities in oxygenation, ventilation, cardiac output, blood pressure, and acidosis, are important in the prevention and effective management of acute renal failure in neonates with septicemia (10).
There are some limitations in this study. First, it had a retrospective design, included medical chart review, and did not contain all the necessary information. Second, it was a single-center study with a relatively small sample size. Therefore, larger multicenteric prospective studies are suggested for a better understanding of the risk factors and independent outcomes of AKI in neonates with septicemia.