1. Background
Mycoplasma pneumoniae is one of the most important etiological agents of respiratory tract infections (1). Respiratory tract diseases in children are one of the leading causes of death worldwide (2).
Mycoplasma pneumoniae and Chlamydophila pneumoniae are causes of 5% to 10% of cases of upper respiratory tract infections including, tracheobronchitis, pharyngitis, laryngitis, and sinusitis (3). It was shown that in community-acquired pneumonia (CAP) cases, M. pneumoniae was isolated from up to 40% of samples, and more than 18% of samples from hospitalized children (1). Mycoplasma pneumoniae infections appear in an epidemic pattern every few years (every 3 to 7 years), yet may also remain totally asymptomatic (4-6). Moreover, M. pneumoniae infection can occur before the onset of asthma, which is accompanied by worsened symptoms, and even deteriorates the management of asthma (7).
It has widely been accepted that M. pneumoniae may be rather uncommon in children aged less than 5 years, and most common in 5- to 15-year-old children with a decreasing rate in adulthood (8). However, newer information showed more broad age spectrum, especially in less than 5-year-old children (9). Also, the prevalence of M. pneumoniae infection in children less than 4 years old was documented by the report of 2010 to 2011 European epidemic data (10). In a research in Rasht, M. pneumoniae prevalence was 1% in 261 children with acute respiratory infections (11). In another study from Shiraz, 10% of children with acute lower respiratory infection had positive polymerase chain reaction (PCR) results for M. pneumoniae (12). Due to the fastidious nature of M. pneumoniae, culturing, and PCR methods are time consuming and expensive; therefore, diagnosis of the organism is usually done by serological tests (13).
Primary infection with M. pneumoniae indicated by IgM antibody increases during the first weeks, which can persist for a few months from the beginning of infection (14). About two weeks after primary infection, IgG antibody appears (8, 14). Detection of M. pneumoniae specific IgM could be a marker of recent infection, particularly in children (15). As a probable result of repeated exposures to M. pneumoniae, adults may not produce a measurable IgM antibody. Thus, it seems that determining the IgM and IgG antibodies in pediatric population, is a valuable epidemiological tool to assess the burden of recent or previous exposures to the bacteria.
2. Objectives
This study was conducted to determine the seroprevalence of Mycoplasma pneumoniae specific IgM and IgG antibodies in asymptomatic 5- to 6-year-old children in Tehran, Iran.
3. Methods
3.1. Subjects and Study Design
This cross sectional study was conducted within the summer season of year 2010 in Tehran, North of Iran. In total, 291 children were enrolled in the study in a sequential method. Tehran city was divided to five regions based on its 22 districts: northern, eastern, central, western, and southern districts. One health care center was chosen from each region and the sample size in each region was approximately equal. The inclusion criteria were age of 5 to 6 years old, and having been referred to health care centers as part of their vaccination program. All children were healthy and had no symptoms. Children were excluded from this study if they had any chronic disease, especially autoimmune diseases, history of blood transfusions or immunosuppressive therapy. This study was in accordance with the declaration of Helsinki and was approved by the ethics committee of Shahid Beheshti University of Medical Sciences. An Informed consent was obtained from children’s parents before the study initiation.
3.2. Sampling and Antibodies Detection
Blood samples were obtained from each child and the serum was separated and stored frozen at -70°C for later antibody assay. Detection of IgM and IgG antibodies against M. pneumoniae was performed on specimens using commercial qualitative Enzyme Linked Immunosorbent Assay (ELISA) kits (IBL-GmbH, Hamburg, Germany), according to the manufacturer’s instructions. A titer of IgM and IgG antibodies greater than 12 U/mL was considered as positive, a value less than 8 U/mL as a negative result, and the borderline titers (8 to 12 U/mL) accounted as negative.
3.3. Statistical Analysis
The results were presented as descriptive statistics in terms of relative frequency. Statistical analysis was performed using the SPSSTM software, version 21.0 (IBM Corp., USA). Chi-square was used to analyze the results and P < 0.05 was considered as statistically significant.
4. Results
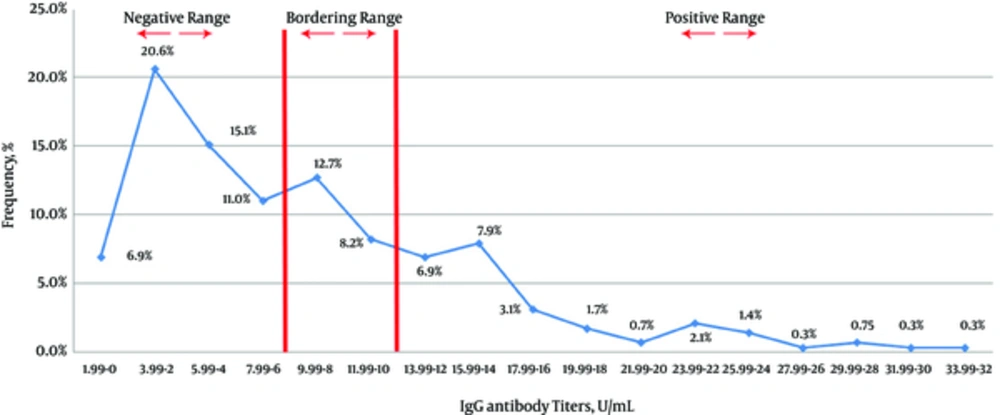
Of the 291 children in the present study, 156 (53.6%) were female and 135 (46.4%) were male. Overall, 73 (25.1%) of the serum samples were positive for M. pneumoniae specific IgG. Furthermore, 156 (53.6%) and 62 (21.3%) of the samples were negative and equivocal for M. pneumoniae-specific IgG, respectively. The mean ± SD M. pneumoniae specific IgG positivity was 17.2 ± 4.9 U/mL, with a range from 12.1 to 33 U/mL. The frequency of positivity and mean of titers for M. pneumoniae specific IgG antibody among males and females was 31 (23%), 17.4 ± 5.5 U/mL (95% CI 16.77 - 18.03) (range from 12.2 to 33 U/mL) and 42 (26.9%); and 17.1 ± 4.4 U/mL (95% CI 16.6 - 17.6) (range from 12 to 28 U/mL), respectively. There was no significant difference between the positivity and mean IgG titers. The distribution pattern of M. pneumoniae specific IgG titers for studied children is demonstrated in Figure 1.
All tested serum samples for M. pneumoniae specific IgM had negative results. The overall mean of IgM titers was 3.4 ± 1 U/mL (ranged from 2 to 6 U/mL). In terms of specific IgM mean titers, no significant difference was observed between male and female children (P ≥ 0.05).
5. Discussion
This study confirm that in preschool children no case with positive IgM anti-M. pneumoniae results was detected, which can be concluded that the majority of children had not been exposed to M. pneumoniae over 6 months to 1 year prior to sampling.
Using a single serum specimen for detection of acute infection with M. pneumoniae had a sensitivity of 31.8% in Japanese children with pneumonia. The number increased to 88.6% when paired acute and convalescent sera were utilized (16). Our study sampling was carried out during the summer season. The temperature could have various effects on the frequency of some respiratory tract pathogens (17). Mycoplasma pneumoniae IgM in winter was found significantly higher compared to other seasons (18).
In a study on patients with respiratory tract infection in Tabriz, presence of M. pneumoniae infection was detected by culture, ELISA, and PCR methods, and the prevalence of M. pneumoniae with these tests was 6.15%, 5.3%, and 2.01%, respectively. Overall, 53% of patients had elevated IgG level and only 5.3% had IgM positive test results, which indicated active M. pneumoniae infection (19). In a study in Greek, 225 children, who were hospitalized for respiratory tract infections were assessed regarding the presence of IgG and IgM antibodies by ELISA (20). Mycoplasma pneumoniae infection was diagnosed in 25 (11.1%) cases, as the second most common detected microorganism. In individuals with respiratory tract symptoms, the proportion of M. pneumoniae infection was significantly higher in the age group of 8 to 14 years than 0 to 3 years old group (20). In a prospective study with 93 admitted children in India, IgM antibody against M. pneumoniae was detected in 23.96% of symptomatic children by ELISA (21). The highest rate of infection was found in the 2 to 5 and 5- to 10-year-old groups (21). Another explanation for the negative rate of IgM antibody against M. pneumoniae in our study could be the type of selected children, who were asymptomatic while in the majority of the mentioned studies the enrolled population was those with respiratory tract infection symptoms.
One important factor in the seroprevalence pattern of M. pneumoniae could be the age of the tested individuals. Several reports indicated that M. pneumoniae infection mostly affects school-aged children and young adults and patients above 5 years old than under 5 years old (22, 23). In another study, in a non-epidemic season, done by serology on healthy Finnish population, seroprevalence of M. pneumoniae was shown to increase in 2- to 4-year-old children and seroprevalence to M. pneumoniae increased in adolescence, yet, the increase leveled off at about 40% to 50% in adulthood (24). A study that investigated over 500 hospitalized children (the mean age was 4.49 years, ranging from 2 weeks to 14 years) with lower respiratory tract infections for M. pneumoniae using molecular and serological methods in Tunis, found that 61.5% of diagnosed cases, were under 5 years old (25).
IgG against M. pneumoniae typically tracks the IgM response 2 weeks later and can remain positive after many years of infection (8). A M. pneumoniae infection was confirmed through high IgG antibody titers (26). Our results showed that 25% of preschool aged children had M. pneumoniae IgG that means they had at least one episode of infection with the organism in their lifetime.
In our study, gender did not have any significant association with rate of M. pneumoniae IgG positivity. Similar to our findings, Touati et al. stated that despite the greater number of girls with positive anti-M. pneumoniae IgG than boys in Tunisian patients with an acute M. pneumoniae infection, there was no significant relationship between gender and IgG positivity (25). Finally, our study had some limitations. First, using single serum specimen in the present study to detect M. pneumoniae IgM may miss some true recent infections.. Second, our studied subjects were asymptomatic children, expected to have lower rate of recent infection with M. pneumoniae.
In summary, beside the limitations, our findings showed frequency of recent and previous exposure to M. pneumoniae infection among asymptomatic children aged from 5 to 6 years old in our region. Moreover, findings of such surveillance studies could provide useful insights to compare our situation with others.