1. Background
Escherichia coli incorporated more than half of the underlying cause of urinary tract infection (UTI) and breakthrough UTIs (1). Escherichia coli carries shiga toxin and might cause hemolytic uremic syndrome (HUS). It is characterized as microangiopathic hemolytic anemia, thrombocytopenia, and a leading cause of acute kidney injury to infants and children (2, 3). It is associated with high morbidity and mortality, especially in underdeveloped countries. Furthermore, STEC is the most common cause of post-infectious HUS. Patients with urinary tract infection are at the risk of renal scar (4-6) and hypertension (7). Furthermore, it is associated with urinary tract abnormalities. For this reason, patients will be routinely screened (8) for the existence of vesicoureteral reflux (9), urinary tract obstruction, congenital abnormalities of kidney and urinary tract, or voiding dysfunction (10, 11). Furthermore, STEC-producing E. coli are rarely consider in hospitalized patients with UTI (12). There are some studies that have investigated antibiotic resistance gene in this microorganism (12, 13). Nevertheless, there is no exact number of the prevalence and incidence of STEC uropathogen in Iran. However, patients with severe pyelonephritis would never be assessed routinely for shiga toxin harboring. Laboratories are not aware of the importance of serotyping of uropathogenic E. coli, so much the worse for STEC. The aim of this review was to determine the extent to which STEC was incorporated in E. coli as a uropathogen in Iran.
2. Methods
2.1. Protocols and Registration
The preferred reporting items for systematic reviews and meta-analysis (PRISMA guidelines) were used to run this review (14). The protocol was registered on PROSPERO (CRD42016033019) (15) and published (16).
2.2. Eligibility Criteria
The inclusion criteria were presence of urinary tract infection investigated for STEC contamination in Iran, with no limitation of age and language, and assessed between 1985 and 2017. The settings were laboratories, hospitals, outpatient facilities, day-care centers, and military institutions. Identification of STEC was by Sorbitol-MacConkey (SMAC) agar, serotyping by any of the recognized techniques, such as agglutination tests, and/or confirmed by PCR for shiga toxins.
The researchers searched PubMed, Google Scholar, OVID, SCOPUS, Web of Sciences, MagIran, health.barakatkns.com, SID.ir, dociran, PDFiran, Ganj.irandoc, and abstract books of congresses. Besides checking for bibliography of included articles for further references, the researchers made contact through email with the authors if the data was missed in the report. The following key words of Shiga toxigenic, enterohemorrhagic, verotoxin Escherichia coli, urinary tract infection, hemolytic-uremic syndrome, E. coli O157:H7, and human were used for the search and the identical Farsi key words were used for Iranian databases.
Study selection and data collection process:
Three reviewers (AA, SN, RN) individually reviewed the abstracts to designate the pertinent studies, in case of disparity “NH” was a determiner. The STROBE statement was used to assess the quality of studies as mentioned in the protocol (15-17). Biography of study, language of study, center of study, type of study, period of study, sample size, patient information (age, disease, and gender), technique of STEC identification, type of STEC, and funding sources was extracted. Hoy et al. (2012) bias appraisal checklist was used to evaluate the articles (18).
2.3. Data Synthesis and Analysis
The prevalence was calculated from the number of samples confirmed for STEC and total population of each study. The incidence was calculated from the number of STEC samples to the population of the city of study. The statistics were taken from population and housing census of the statistical center of Iran. Moreover, prevalence was pooled using the random effects model with the reverse variance technique of DerSimonian and Laird for between-study variance estimation. Subsequently, confidence interval as the normal approximation interval between the 95% level was computed. Chi-squared tests and the I-squared statistic to rate heterogeneity between the studies in effect measures was used (I-squared value of > 50% denotes considerable heterogeneity).
Provisional Search strategy for Pubmed:
(“urinary tract infections”[MeSH Terms] OR (“urinary”[All Fields] AND “tract”[All Fields] AND “infections”[All Fields]) OR “urinary tract infections”[All Fields] OR (“urinary”[All Fields] AND “tract”[All Fields] AND “infection”[All Fields]) OR “urinary tract infection”[All Fields]) OR “pyelonephritis”[MeSH Terms] OR “pyelonephritis”[All Fields] OR “cystitis”[MeSH Terms] OR “cystitis”[All Fields] AND shiga toxin[All Fields] OR “enterohaemorrhagic e coli”[All Fields] OR “Escherichia coli o157”[MeSH Terms] OR (“Escherichia”[All Fields] AND “coli”[All Fields] AND “o157”[All Fields]) OR “Escherichia coli o157”[All Fields] OR “enterohemorrhagic e coli”[All Fields] OR “enterohemorrhagic Escherichia coli”[MeSH Terms] OR (“enterohemorrhagic”[All Fields] AND “Escherichia”[All Fields] AND “coli”[All Fields]) OR “enterohemorrhagic Escherichia coli”[All Fields]
OR “enterotoxigenic Escherichia coli”[MeSH Terms] OR (“enterotoxigenic”[All Fields] AND “Escherichia”[All Fields] AND “coli”[All Fields]) OR “enterotoxigenic Escherichia coli”[All Fields] OR “enterotoxigenic E coli”[All Fields] OR (“haemolytic uraemic syndrome”[All Fields] OR “hemolytic-uremic syndrome”[MeSH Terms] OR (“hemolytic-uremic”[All Fields] AND “syndrome”[All Fields]) OR “hemolytic-uremic syndrome”[All Fields] OR (“hemolytic”[All Fields] AND “uremic”[All Fields] AND “syndrome”[All Fields]) OR “hemolytic uremic syndrome”[All Fields]) OR (“shiga toxins”[MeSH Terms] OR (“shiga”[All Fields] AND “toxins”[All Fields]) OR “shiga toxins”[All Fields] OR “verocytotoxin”[All Fields]) AND (“Escherichia coli”[MeSH Terms] OR (“Escherichia”[All Fields] AND “coli”[All Fields]) OR “Escherichia coli”[All Fields] OR “e coli”[All Fields])
AND (“iran”[MeSH Terms] OR “iran”[All Fields])
3. Results
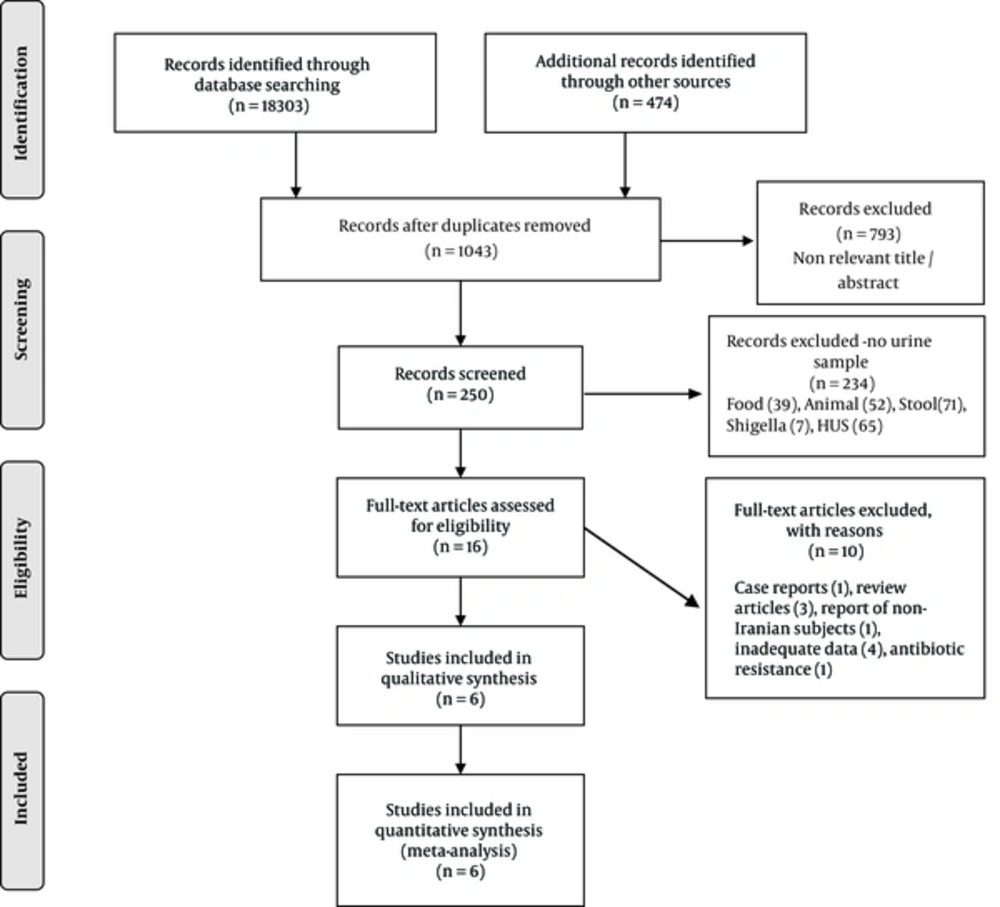
At the preliminary search, 250 articles were detected; 16 studies were relevant (Figure 1). After reviewing the abstract, a total of six potentially relevant articles containing 1,111 positive E. coli urine cultures were eligible for the final analysis. Amongst them, 32 were positive for STEC. The design was cross sectional. Two studies had poor and the rest had good/fair quality. One study evaluated mixed groups of patients with either diarrhea or urinary tract infection (19). Median duration of studies was 10 months (range 5 to 12 months) (Table 1). Detail of extracted data is shown in Table 2.
| Study ID | Age, y/Gender/Population | E. coli | STEC | Date | Duration Study, mo | Setting, Location | Identifying Methods | Design | Quality/Bias |
|---|---|---|---|---|---|---|---|---|---|
| Abbasi and Tajbakhsh (20) | 1 - > 49; F: 51, M: 25/Symptomatic UTI | 76 | 3 | 2014 | 12 | Outpatient, Shahrekord | PCR/LAT | CS | Good/Low |
| Mansouri et al. (21) | UA/Symptomatic UTI | 146 | 0 | 2010 - 2011 | 12 | Outpatient, Lorestan | PCR | CS | Poor/Low |
| Adeli et al. (22) | Age-UA/F: 117, M: 29/UTI - hospitalized | 100 | 3 | 2001 - 2002 | 6 | Hospital, Lorestan | PCR | CS | Poor/Low |
| Nazemiet al. (23) | UA/UTI | 100 | 16 | 2010 - 2011 | 12 | Laboratory, Iran | PCR | CS | Good/Low |
| Navidinia et al. (24) | 1 - 12/Gender-UA/Suspected to UTI | 378 | 9 | 2008 - 2009 | 8 | Hospital, Tehran | PCR | CS | Good/Low |
| Aghaee (19) | UA/Urine culture of patients with diarrhea | 311 | 1 | 1999 - 2000 | 5 | Hospital, Khoramabad | PCR | CS | Good/Low |
Abbreviations: CS, cross sectional; LAT, latency-associated transcript; PCR, polymerase chain reaction; STEC, Shiga toxin-Escherichia coli.
Abbreviations: n, number; STEC, Shiga toxin-Escherichia coli; Stx, shiga toxin.
aFarsi language.
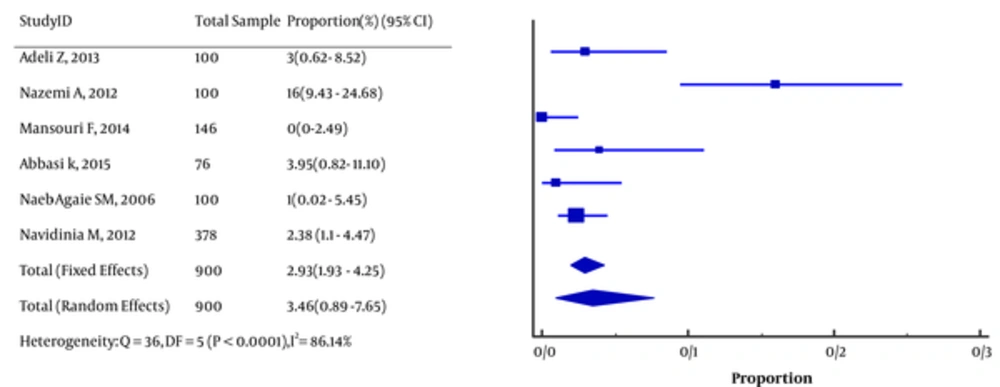
The pooled prevalence of STEC in E. coli-associated UTI was 3.46 (95% CI, 0.89 to 7.65; I2 = 93.6%; Figure 2). Virulence factors were stx1 (n = 11), stx2 (n = 9), stx1, 2 (n = 6), stx1eaeA (n = 1), and stx2 eaeA (n = 2).
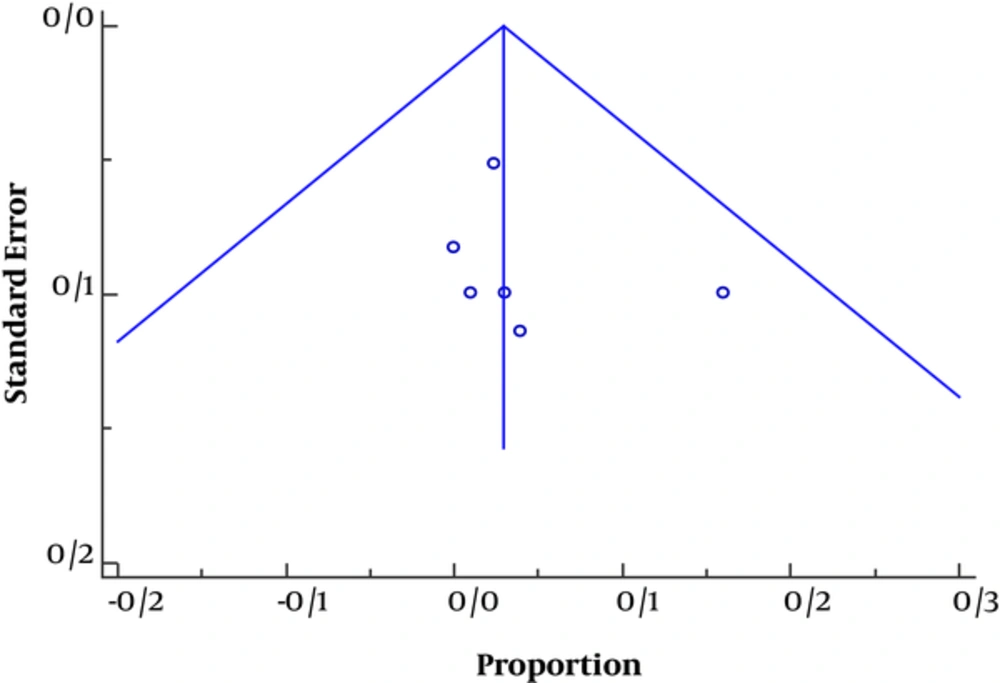
Funnel plot (Figure 3) was asymmetric toward positive axis in favor of publication bias and population heterogeneity and methodology design, thus the studies with positive results were underestimated.
4. Discussion
To the best of the author’s knowledge, this is the first comprehensive report of uropathogen STEC in Iran. Data from six cross–sectional studies with good to poor quality of acceptance and low to moderate of bias were used. The prevalence of urinary STEC was approximately 3.46% in E. coli positive UCs. There is little evidence regarding the correlation between STEC uropathogen and HUS or Thrombotic microangiopathy (TTP) (25-29).
Only one study performed a serotype for O157 (23). The dominant virulence factors were stx1 and stx2. Surveying of literature revealed the occurrence of non-O157 UTI associated with HUS. The responsible serotypes were O113:H21, O6:H1 (30), and O138:H- (31). In addition, in diarrhea negative HUS, other foci, such as urinary system, should be assessed for STEC infection (32). It has been suggested that UTI-associated HUS should be investigated for verotoxin-producing E. coli (33). Because of high infectivity of this pathogen even with low dose of this organism, the physician should be well informed to consider this possibility and check VTEC in cases with aggressive manifestation of urinary tract infection, diarrhea negative HUS, or TTP. The central laboratories should be enlightened to be assembled to investigate both O157 and non-O157 STEC in suspicious victims.
Limitations: The heterogeneity of studies was one of the major drawbacks. None of the studies followed the patients for occurrence of STEC complications, such as hemorrhagic colitis or hemolytic uremic syndrome, changes in blood cells, or kidney function. None of the studies reported the outcome of affected and non affected cases. Small sample size was another limitation of the study.