1. Background
Among infectious diseases, pneumonia is the leading cause of childhood mortality and the most common cause of hospitalization (1, 2). The mortality rate due to acute respiratory infections, especially pneumonia, is estimated to be two million per year, which is the highest in developing countries, including Iran (1). The WHO report indicates that ALRIs account for 19% of mortality in children under the age of five (3).
The global prevalence of vitamin D deficiency among children in different parts of the world is estimated to be between 30% and 90% depending on diet, environmental conditions, and latitude (4). In limited studies conducted in Iran, the prevalence of vitamin D deficiency in children has ranged from 81.3% and 37.9% based on age, gender, and living place (5-7), which is also linked to the amount and type of vitamin D intake.
Practically, 25(OH)D and 1,25(OH)2D are surrogate markers of reservoir and active forms of vitamin D, respectively. Although measuring serum level of 25(OH)D is generally considered the best way to determine the status of vitamin D in humans (8-10), 1,25(OH)2D has the ultimate role of vitamin D in the body, including in the immune system. Due to a relatively short half-life (12 to 36 hours) of 1,25(OH)2D compared to 25(OH)D (3 weeks) (11, 12), it is probably better to measure the serum level of 1,25(OH)2D instead of 25(OH)D for a better prediction of the vitamin D role in decreasing the severity and improving prognosis of patients with ALRIs.
Different studies have had different results regarding vitamin D deficiency and its effects on respiratory infections (13-17). Some studies have shown the potential protective effect of vitamin D against ALRIs (13, 15, 16), while other studies have shown that vitamin D supplementation to infants cannot prevent pneumonia (17).
Researches, which evaluate the association of 25(OH)D and severity of respiratory tract infection, are scarce and findings are controversial. One study conducted in Iran in 2016 - 2017 (18), declared a positive relationship between low level of 25(OH)D and severity of pneumonia. However, some studies found no such association, for example, the study of Kim et al., in a tertiary referral hospital in South Korea (19).
Confirming this argument, serum level of 1,25(OH)2D is associated with an increase in symptoms and mortality in patients with HIV infection, however, low 1,25(OH)2D levels did not appear to be related to vitamin D deficiency (20). Another study by Zittermann et al. showed that low serum levels of 1,25(OH)2D in patients with heart disease is associated with an increase in death due to coronary heart disease, heart failure, hypertension, diabetes, and renal failure (21). Powe et al. also recommended that both serum level of 1,25(OH)2D and 25(OH)D should be checked to understand the complex effects of vitamin D metabolism (22).
As far as we know, in our country, despite the low level of vitamin D, there have been few studies on the association of ALRIs with vitamin D level. To the best of our knowledge, this is the first study conducted to investigate the serum level of 25(OH)D and 1,25(OH)2D in children with ALRIs, simultaneously.
2. Objectives
Considering the importance of community-acquired pneumonia in terms of pathogenicity, mortality and its imposed costs, and the controversy about the role of vitamin D deficiency in ALRIs in children and the relation between the severity of the disease and vitamin D serum level, this study was conducted to simultaneously study the serum level of 25(OH)D and 1,25(OH)2D in children with ALRIs and its association with severity of disease.
3. Methods
The simple random sampling technique was used in this hospital-based cross-sectional study to select 110 children among children aged two months to five years admitted with fever, cough, and tachypnea to the Imam Hossein Children’s Hospital (the major tertiary referral hospital in Isfahan province, Iran). Parents’ consent for their children’s participation in the study was also considered as mandatory. The study was approved by the Ethics Committee of Isfahan University of Medical Sciences (≠396031). Patients excluded from the study were those with underlying medical disorders including: history of chronic pulmonary disease (such as CF, asthma, etc.), aspiration pneumonia, anatomical defects of airways or lungs, chronic kidney disease, swallowing disorder, congenital heart disease, gastroesophageal reflux, malabsorption, jaundice, neuromuscular disease (such as SMA, etc.), a known immunodeficiency disease, and a history of receiving drugs such as: Phenobarbital, phenytoin, carbamazepine, isoniazid, theophylline, rifampin.
Explaining the purpose of the study to parents and obtaining written consent from them, information and findings from the clinical history and the physical examination were listed in the designed checklist.
World health organization’s (WHO) definition of ALRIs was used for definition of ALRIs or pneumonia, i.e. cough or difficulty in breathing with fast breathing (≥ 50 breaths/min in a child aged two to 11 months or ≥ 40 breaths/min in a child aged one to five years or ≥ 30 breaths/min in a child aged > 5 years) or chest indrawing (23). Bronchiolitis was considered for children younger than two years old, with a viral upper respiratory tract prodrome, followed by increased respiratory effort and wheezing (24).
As there is no clinical diagnostic criterion for the definitive diagnosis of bacterial pneumonia, and a series of clinical manifestations and radiological findings are required for a definitive diagnosis (25), the diagnosis of bacterial pneumonia was based on the presence of consolidation, opacity, or infiltrate on a chest radiograph and standardized radiographic definition of pneumonia based on WHO criteria in children who have taken chest radiography (26-28). For the cases without chest radiography, a set of symptoms and laboratory findings were used to differentiate the two types of bacterial and viral pneumonia. These symptoms and findings included persistent or recurrent fever > 38.5°C, increased respiratory rate, chest indrawing, hypoxia, leukocytosis > 20,000, and neutrophilia, and most importantly, the clinical course of patients during admission and course of the disease (26, 29).
According to criteria provided by Seiden et al. and Dooley et al. the severity of infection in patients with pneumonia and bronchiolitis was classified in three categories: mild, moderate and severe (30, 31).
Population study weights and heights were measured with standard methods and WHO chart were used for interpretation.
To determine the serum level of 1,25(OH)2D and 25(OH)D in patients, 2 mL of the blood clot was collected in the test tube and sent to the lab. Sampling was performed either at the time of admission or at most within the first 24 hours of admission. The serum of patients was stored in a freezer at -20°C. Serum level of 1,25(OH)2D and 25(OH)D were measured by HPLC method using commercial kits (ZellBio GmbH, Germany), according to the manufacturer’s instructions.
We considered a serum level of 1,25(OH)2D and 25(OH) greater than or equal to 30 ng/mL as sufficient, the serum level of 21 - 29 ng/mL as deficient and serum level below 20 ng/mL as severely deficient.
The data were analyzed by SPSS software version 23 and analyzed with the significance level of 0.05. To describe the quantitative data, the mean and standard deviation were used. To characterize the qualitative data, a frequency index and frequency were used; we used variance analysis, chi-square, independent t-test. Also linear regression test was used for association of 1,25(OH)2D, 25(OH)D and severity of ALRIs
4. Results
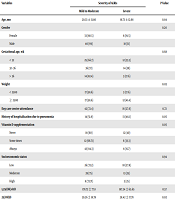
Eleven out of 110 children involved in the study were excluded from the study, according to exclusion criteria, and the study continued with 99 children. Demographic data of children was presented in Table 1.
| Variables | Severity of ALRIs | P Value | |
|---|---|---|---|
| Mild to Moderate | Severe | ||
| Age, mo | 20.33 ± 13.80 | 19.73 ± 12.68 | 0.84 |
| Gender | 0.20 | ||
| Female | 33 (80.5) | 8 (19.5) | |
| Male | 40 (69) | 18 (31) | |
| Gestational age, wk | 0.68 | ||
| < 32 | 25 (66.7) | 12 (33.3) | |
| 32 - 36 | 36 (72) | 14 (28) | |
| > 36 | 14 (82.4) | 3 (17.6) | |
| Weight | 0.03 | ||
| < 2500 | 17 (58.6) | 3 (17.6) | |
| ≥ 2500 | 17 (58.6) | 12 (41.4) | |
| Day care centre attendance | 42 (72.4) | 16 (27.6) | 0.72 |
| History of hospitalization due to pneumonia | 14 (51.9) | 13 (48.1) | 0.05 |
| Vitamin D supplementation | 0.05 | ||
| Never | 18 (60) | 12 (40) | |
| Some times | 12 (66.71) | 6 (33.3) | |
| Always | 43 (84.3) | 8 (15.7) | |
| Socioeconomic status | 0.94 | ||
| Low | 26 (72.2) | 10 (27.8) | |
| Moderate | 39 (75) | 13 (25) | |
| High | 8 (72.71) | 3 (25) | |
| 1,25(OH)vitD | 178.72 ± 77.8 | 187.24 ± 65.65 | 0.57 |
| 25(OH)D | 39.26 ± 18.78 | 28.42 ± 17.78 | 0.02 |
A total of 7% of children had a weight for height Z. score of ≤ -2.5, -3. None of children had severe malnutrition (Z. Score ≤ -3). The percentage of breastfeeding and formula feeding in the study group were 40.4 and 33.3, respectively and 26.2% of children were fed on both. Regarding vitamin D supplementation in the first two years of life, 51% of children had a sufficient intake, 18% had an insufficient intake, while 30% didn’t receive any vitamin D.
According to the findings, 36.4% (number = 36, male = 22, female = 14) of patients had bronchiolitis, 39% (number = 39, male = 18, female = 21) had viral pneumonia, and 24% (number = 24, male = 19, female = 24) had bacterial pneumonia.
Child with Bronchiolitis, viral pneumonia, and bacterial pneumonia had no significant difference in levels of 25(OH)D and 1, 25(OH)2D (Table 2).
| Groups of ALRIs | Mean ± SD | P Value | |
|---|---|---|---|
| 25(OH)D | Bronchiolitis | 39.55 ± 18.71 | 0.36 |
| Bacterial pneumonia | 32.82 ± 18.60 | ||
| Viral pneumonia | 35.23 ± 20.75 | ||
| 1,25(OH)2D | Bronchiolitis | 177.49 ± 10.36 | 0.24 |
| Bacterial pneumonia | 176.03 ± 95.5 | ||
| Viral pneumonia | 173.35 ± 89.14 |
Levels of 25(OH) D and 1,25(OH)2D in Lower Respiratory Infections (ALRIs)a
T-test analysis showed child with lower level of 25(OH)D had significant sever ALRIs (P = 0.02) (Table 1).
Linear regression analysis showed a lower level of 25(OH)D significantly associated with severe lower respiratory syndromes (B = -0.7, P = 0.00), unlike, 1, 25(OH)D, which was not significantly associated with more severe ALRIs (B = -0.01. P = 0.99) (Table 3).
5. Discussion
According to data in the present study, low serum level of 25(OH)D was significantly correlated with increasing ALRIs severity.
In the review study conducted in 2014, 13 out of 18 studies revealed that vitamin D deficiency was common in children with respiratory infections, and in four other studies, there was no significant difference in vitamin D level between patients and control groups (9). The result of this study, which was conducted in a country with vitamin D deficiency is prevalent among children, which is in line with studies proving the positive effect of vitamin D deficiency in increasing the risk of pneumonia in children.
On the other hand, the serum level of active form of vitamin D [1,25(OH)2D] in children with a mild ALRIs was lower than severe cases. This difference was not statistically significant. While Pletz and colleagues found a modest positive relationship between serum level of 25(OH)D and 1,25(OH)2D in 300 adult patients with community-acquired pneumonia; only serum level of 1,25(OH)2D had a significant negative correlation with pneumonia severity (32). This difference may be due to confounding factors such as age (recent study was in adults) and other underlying conditions, however, there is no linear relationship between serum 25(OH)D level and serum level of 1,25(OH)2D in both studies, confirming the complexity of vitamin D metabolism and the need for further studies to determine the mechanism through which vitamin D affects the immune system.
According to the results of this study, low serum level of 25(OH)D has an effect on the increase of ALRIs severity. Pletz and colleagues found that gender and 25(OH)D level in adults with pneumonia were effective in increasing ALRIs (32). In this study, as in the study of Hosseininejad and colleagues, factors such as age, number of admissions due to pneumonia, and level of vitamin 1,25(OH)2D were considered ineffective (33).
In our study, there was neither a significant relationship between the severity of the infection and the type of nutrition nor between the degree of malnutrition and the length of hospitalization.
One study in Tanzania found that exclusive breastfeeding was associated with a significant decrease in the risk of respiratory disease in the first six months of life among 666 children (34). However, a cohort study from South Africa reported higher mortality in infants who were exclusively breast fed than in infants who were mixed fed (35). In our country and some other developing countries, ineffectiveness of breast feeding on severity of infections in infants may be due to vitamin D insufficiency in mothers. It also may be due to the fact that effectiveness of breast feeding on severity of infections, mostly seen in the first two years of age but target groups of our study, were children under five years old.
Factors unrelated to pneumonia severity may influence the hospitalization decision. However, studies in adults with pneumonia indicate that site of care decisions vary considerably by provider and that risk for severe outcomes is often overestimated (36).
In most studies, a significant relationship between FTT and severity of infections was found (37). However, we did not see this relationship, which may be due to low numbers of FTT cases in our study.
In this study, the decrease in serum level of 25(OH)D caused a significant increase in some ALRIs’ severity criteria such as ICU admission, decreased arterial oxygen saturation, prolonged capillary refill time, more duration of oxygen therapy, and more duration of hospitalization. These results are partially consistent with the results of Zhang et al. 2016 in China, confirming inverse correlation between serum level of 25(OH)D and ALRIs’ severity criteria in children with ALRIs such as respiratory rate, cyanosis, chest indrawing, feeding intolerance, and the need for oxygen (38). In 2007 and 2008 a Canadian study of children with ALRIs, had no significant difference between patients and control group regarding their vitamin D serum level, however, most of the children admitted to ICU with ALRIs were deficient in vitamin D (39). In the present study, decreasing serum level of 25(OH)D significantly increases the incidence of admission of children with ALRIs in ICU.
Although the findings of this study and most observational studies indicate a low serum level of 25(OH)D in children with pneumonia, the addition of vitamin D supplementation to children’s or adolescents’ diets in some cases does not reduce the incidence of respiratory infection. This may be due to several reasons. In most of these studies, the serum level of 25(OH)D have not been measured before the onset of supplementation, and presumably, the administration of vitamin D in people with normal serum level of vitamin D cannot help reduce the incidence of respiratory infections. On the other hand, the dose and mode of administration of vitamin D in these individuals have not been the same, and a universal protocol has not been determined in this case (40). However, in our study, we did not address the effect of vitamin D supplements on reducing the incidence and severity of ALRIs, but future studies are needed to be done on the effect of supplementation with vitamin D in reducing the incidence and severity of ALRIs, while serum level of both 1,25(OH)2D and 25(OH)D are measured in children before administering vitamin D.
The strengths of the present study are the use of valid criteria for the diagnosis and differentiation of ALRIs types and the assessment of the severity of ALRIs. In addition, this study, unlike the previous ones, has concurrently measured the level of reservoir form and active form of vitamin D in children with ALRIs to help clarify the role of vitamin D in the ALRIs.
One of the limitations of this study is lack of a healthy control group, and the other one is not checking the changes of serum level of reservoir form and active form of vitamin D before the onset of the ALRIs and in their courses.