1. Background
Hemophagocytic lymphohistiocytosis (HLH) is an immune-dysregulatory disorder with potentially lethal complications (1). Its incidence is reported as one case per 3000 inpatient admissions in pediatric hospitals. Since the disease mostly occurs during infancy and early childhood, it is believed that HLH has a genetic etiology (2). Due to hypercytokinemia and excessive activation of T-lymphocytes and macrophages, children with HLH are profoundly immunosuppressed. The critical regulatory pathways are responsible for HLH due to the neutralization of immune/inflammatory responses. In such patients, the natural interaction between innate and adaptive immune responses is altered by pathologic genetic defects (3).
Although HLH has a clear pattern caused by pathologic inflammation, it can appear in different clinical circumstances. Prolonged fever, hepatosplenomegaly, pancytopenia, and coagulopathy are the main symptoms of the disease. The other signs include rash, lymphadenopathy, and cerebrospinal fluid (CSF) pleocytosis (2).
It is known that diagnosis is the first essential step to achieve an effective treatment. The HLH diagnosis is a challenging measure owing to its rarity and different presentations. Despite the fact that the HLH diagnostic criteria are very important to diagnose this disorder, they do not reflect all clinical or laboratory features of such patients. For instance, hepatic or neurologic findings are common in patients with HLH, which can present with different severity degrees. The HLH diagnosis is often delayed due to various factors such as the rare occurrence of the disease and intricacy of the diagnostic criteria (4, 5). Moreover, the diagnosis may be challenging in some circumstances. It is noteworthy that the disorder could be triggered by many infections, and infectious complications are the main cause of morbidity and mortality in such patients (5).
Regarding the rarity of this condition, the assessment of the factors affecting HLH is a matter of fundamental importance. The identification of the clinical patterns of this disorder and its underlying pathophysiology can facilitate the implementation of early and accurate diagnosis.
2. Objectives
The present study aimed at determining the clinical features, etiologies, and the rate of HLH mortality among Iranian children.
3. Methods
The current study was performed on children with HLH who referred to the Pediatric Ward of Imam Reza Hospital, Mashhad, Iran during nine years from 2010 to 2018. The study protocol was approved by the Ethics Committee of Mashhad University of Medical Sciences. The data collection tool included items on clinical presentations, physical examination, laboratory data, treatment strategy, and the disease outcome.
The HLH diagnosis was made based on the HLH-2004 Diagnostic Criteria including fever ≥ 38.5°C, splenomegaly, peripheral blood cytopenias (with at least two of the following conditions: hemoglobin < 9 g/dL, platelets < 100000/µL, and neutrophils < 1000/µL), hypertriglyceridemia (fasting triglycerides > 265 mg/dL), and/or hypofibrinogenemia (fibrinogen < 150 mg/dL), low or absent natural killer (NK) cell activity, ferritin > 500 ng/mL, elevated sCD25, and hemophagocytosis in the bone marrow, spleen, lymph nodes, or liver. To diagnose HLH, five of the eight abovementioned criteria should be fulfilled (4). Sepsis was ruled out based on repeated negative blood culture. In cases with neurologic findings, a brain magnetic resonance imaging (MRI) was performed to exclude differential diagnosis and assess the intracranial complications. The cases were divided into two groups of primary and secondary HLH. Primary HLH included familial HLH (FHLH) and the cases with primary immune deficiencies such as the Chediak-Higashi and the Griscelli syndromes. Secondary HLH was referred to cases previously diagnosed with malignancy, infections, or rheumatologic conditions (SLE and SoJIA) (6, 7). Genetic testing was not available in the current study; accordingly, the cases with a sibling diagnosed with HLH were classified as familial cases.
Data were analyzed using independent samples t-test, and the Fisher exact test with SPSS version 20. A P value of less than 0.05 was considered significant.
4. Results
Among the 17 children (11 males and six females) with HLH, the mean age at the disease onset was 4.67 ± 4.6 years (range: 2 months to 13 years). About 94.1% of the patients were Iranian and the rest had other nationalities.
The most common symptoms and signs were fever and splenomegaly, which presented in 100% and 82.5% of the cases, respectively. The mean fever duration was 38.1 ± 54.2 days (range: 7 to 180). The clinical characteristics of the children are presented in Table 1.
| Clinical Characteristics | No. (%) | Clinical Characteristics | No. (%) |
|---|---|---|---|
| Fever | Splenomegaly | ||
| Yes | 17 (100) | Yes | 14 (82.4) |
| No | 0 (0) | No | 3 (17.6) |
| Hepatomegaly | Jaundice (icterus) | ||
| Yes | 13 (76.5) | Yes | 5 (29.4) |
| No | 4 (23.5) | No | 12 (70.6) |
| Rash | Respiratory symptoms | ||
| Yes | 8 (47.1) | Yes | 8 (47.1) |
| No | 9 (52.9) | No | 9 (52.9) |
| Lymphadenopathy | Neurologic signs | ||
| Yes | 7 (41.2) | Yes | 8 (47.1) |
| No | 10 (58.8) | No | 9 (52.9) |
| Headache | Seizure | ||
| Yes | 4 (23.5) | Yes | 3 (17.6) |
| No | 13 (76.5) | No | 14 (82.4) |
| Bulging fontanelle | Ataxia | ||
| Yes | 0 (0) | Yes | 3 (17.6) |
| No | 17 (100) | No | 14 (82.4) |
| Neck stiffness | Insomnia | ||
| Yes | 2 (11.8) | Yes | 1 (5.9) |
| No | 15 (88.2) | No | 16 (94.1) |
| Sixth nerve palsy | Psychomotor retardation | ||
| Yes | 2 (11.8) | Yes | 1 (5.9) |
| No | 15 (88.2) | No | 16 (94.1) |
| Coma | Hemiplegia | ||
| Yes | 2 (11.8) | Yes | 2 (11.8) |
| No | 15 (88.2) | No | 15 (88.2) |
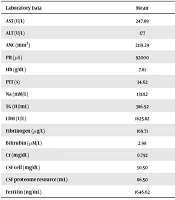
Based on the obtained results of the laboratory data, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were abnormal in 66.7% of the patients. In addition, 23.1%, 90.9%, 40%, and 60% of the cases had abnormal partial thromboplastin time, lactate dehydrogenase level, CSF cell count, and CSF proteome resource, respectively. The brain MRI was performed in 64.7% of the patients. The laboratory data of the children with HLH are presented in Table 2.
| Laboratory Data | Mean | Standard Deviation | Minimum | Maximum |
|---|---|---|---|---|
| AST (U/L) | 247.89 | 281.31 | 57 | 925 |
| ALT (U/L) | 177 | 147.19 | 17 | 409 |
| ANC (mm3) | 2119.29 | 1681.59 | 76 | 6000 |
| Plt (µL) | 92000 | 103286.61 | 7000 | 450000 |
| Hb (g/dL) | 7.81 | 2.33 | 4.6 | 12.5 |
| PTT (s) | 34.62 | 17.38 | 23 | 88 |
| Na (mM/L) | 131.82 | 7.93 | 114 | 141 |
| TG (IU/mL) | 386.92 | 273.9 | 96 | 1035 |
| LDH (U/L) | 1625.82 | 1258.68 | 471 | 4445 |
| Fibrinogen (µg/L) | 169.71 | 143.22 | 55 | 388 |
| Bilirubin (µM/L) | 2.98 | 3.2 | 0.7 | 8.0 |
| Cr (mg/dL) | 0.792 | 0.73 | 0.4 | 3.0 |
| CSF cell (mg/dL) | 30.50 | 48.67 | 0 | 150 |
| CSF proteome resource (mL) | 86.50 | 97.23 | 15 | 345 |
| Ferritin (ng/mL) | 1646.62 | 1465.72 | 62 | 5783 |
Abbreviations: ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; Cr, creatinine; CSF, cerebrospinal fluid; Hb, hemoglobin; LDH, lactate dehydrogenase; Na, sodium; Plt, platelet count; PTT, partial thromboplastin time; TG, thyroglobulin.
Among HLH-2004 Diagnostic Criteria, fever and splenomegaly were the most frequently observed criteria in the current study. The other criteria including cytopenia, hypertriglyceridemia, hypofibrinogenemia, and serum ferritin ≥ 500 ng/mL were observed in 64.7%, 69.2%, 57.1%, and 76.9% of the cases, respectively. In addition, bone marrow hemophagocytosis was detected in 18.8% of the patients. The investigation of soluble CD25 and NK cells activity was not available in the study.
The investigation of etiology in the current study revealed that about 30% of the children had familial form of HLH, 17.5% were previously diagnosed with rheumatologic disorders (one case of systemic lupus erythematosus and two cases of systemic-onset juvenile idiopathic arthritis), and the same rate was afflicted by infectious diseases. Among infectious cases, one patient with the chronic granulomatous disease was infected with Salmonella typhi, one child was infected with Staphylococcus aureus, and another one was a case of endocarditis with Enterobacter agglomerans. Idiopathic factors, and the Chediak-Higashi and the Griscelli syndromes were responsible for the occurrence of HLH in 17.5%, 12%, and 6% of the cases, respectively.
According to the obtained results of the study, 35.3% of the patients with HLH were cured, and about 64.7% (i.e., 11 cases) died. The etiologies and clinical courses of the children that died due to HLH are summarized in Table 3. The mean duration of the disease from onset to death was eight months and the mean length of follow-up in the treated patients was four years.
| Number | Age, y | Etiology | Time to Death, mon | No Response to the Treatment | Initial Good Response, But Relapsed |
|---|---|---|---|---|---|
| 1 | 0.2 | Idiopathic | 2 | + | |
| 2 | 5 | The Chediak-Higashi | 12 | + | |
| 3 | 1 | Familial | 14 | + | |
| 4 | 0.5 | Infection (typhoid) | 1 | + | |
| 5 | 5.5 | Idiopathic | 3 | + | |
| 6 | 13 | Rheumatologic (SLE) | 12 | + | |
| 7 | 1 | Familial | 24 | + | |
| 8 | 0.5 | Familial | 12 | + | |
| 9 | 1 | Familial | 2 | + | |
| 10 | 3 | The Griscelli syndrome | 6 | + | |
| 11 | 5 | Idiopathic | 2.5 | + |
Liver enzymes (AST and ALT) activities were compared between the groups with and without rheumatologic diseases and no statistically significant difference was observed (P > 0.05). In addition, no significant difference was observed between the groups regarding neurological disorders (P > 0.05).
Even though the mean age at the onset was lower in patients that died (3.6 ± 3.7 years) in comparison with survivors (6.5 ± 5.7 years), the difference was not statistically significant (P = 0.31). Similarly, no significant difference was observed in gender, etiology, infection, respiratory disorder, and neurological signs and symptoms between cured patients and the expired ones (P > 0.05). The overall mortality rate of the current study cases was 65%, yet, the comparison of mortality between the two groups of primary and secondary HLH showed no significant differences (P = 0.08).
5. Discussion
Nowadays, there is improved knowledge about the etiology of HLH as a syndromic disorder with a unique pattern. However, persistent efforts are made to identify the genetic and immunologic basis of this syndrome.
Although FHLH, which is an autosomal recessive disorder, is usually reported in the offspring of consanguineous parents, it can occur due to sporadic mutations. FHLH commonly occurs prior to the first year of life, yet it may occur in other age groups (7). In the present study, 60% of the cases were categorized as primary HLH, and FHLH was observed in 45% of the cases with the mean age of less than one year. It seems that the high rate of primary HLH in the current study was due to the high frequency of consanguineous marriage in the Iranian population, which led to the occurrence of rare immunodeficiency diseases in this population (8, 9). No etiologies could be found for HLH in three patients and there was no positive family history for these conditions; thus, they were classified into an idiopathic group under the category of primary HLH.
It is mentioned in the literature that the disease has no predilection for gender. Leow et al., presented 14 children with HLH, among whom eight (57.1%) were male (10). Based on the obtained results of the current study, the male to female ratio was 1.8:1 (55% male), which was inconsistent with the previous studies.
HLH is often associated with viral infections, especially Epstein-Barr virus, and it may less commonly occur due to bacterial, parasitic, or fungal infections (11). Among the current study patients, there was a nine-year-old female with Enterobacter agglomerans endocarditis and HLH syndrome. In this case, antibacterial treatment of endocarditis resolved all the symptoms and signs of HLH. Wang et al., reported the case of a 52-year-old male patient with a history of chronic hepatitis C virus (HCV) infection, fungal endocarditis, and HLH at autopsy (12).
The prevalence of hemophagocytosis in lymphatic tissue ranges 25% to 100%. The diagnosis of HLH does not rely on tissue hemophagocytosis, since this finding is neither sensitive nor specific to HLH (13). In the current study, hemophagocytosis in tissue was observed in less than 20% of the patients. The lower incidence of tissue hemophagocytic syndrome in the current study may be due to the lack of parental permission to perform bone marrow aspiration or lymphatic tissue biopsy.
A ferritin level of > 10000 g/dL is highly specific and sensitive to diagnose HLH. Although ferritin is known as a valuable marker to diagnose HLH, the level of sCD25 correlates with HLH activity more reliably than those of ferritin or other disease indices (14). Moreover, prolonged fever and hepatitis are the two main clinical signs of HLH reflecting immunologic perturbation (15). The investigation of the diagnostic criteria in the current study showed that all the children with HLH were febrile. Transaminase elevation was observed in 35% of the cases, although clinical signs of hepatitis appeared in 30%. Ferritin > 500 ng/mL was observed in more than 75% of the patients, yet none of them presented a ferritin level of > 10000 g/dL.
Treatment of any form of HLH should focus on suppressing the exaggerated immune response (16). According to the HLH-2004 Treatment Protocol, an eight-week therapy with corticosteroids, etoposide, and cyclosporine is recommended. Correction and treatment of the underlying immune defect or underlying disease are also indicated. Stem cell transplantation (SCT) is recommended in certain cases and it can have a curative potential both for FHLH and acquired HLH (17, 18). In the present case series, the patients were treated based on the HLH-2004 Treatment Protocol. As mentioned above, only one patient received antibiotic therapy for the underlying infectious disease (endocarditis) without any need for anti-inflammatory therapy. Additionally, one case with the Chediak-Higashi syndrome underwent hematopoietic stem cell transplantation (HSCT) and the result was successful.
The assessment of mortality in patients with HLH is an issue of paramount importance. Although the mortality rate of is high and the survival of patients with FHLH is two months in the absence of treatment, the overall survival in FHLH significantly improved after the introduction of the allogeneic HSCT (19). The severity and outcomes are more variable in the acquired HLH than in genetic HLH, and mortality rate is higher than 50% (17, 20). In the current study, while the overall mortality rate was 65%, the difference between the two groups of primary and secondary HLH was not significant. This finding could be due to the small sample size in this case series. The higher rate of mortality in the current study cases compared to those of the previous studies could be due to the late referral time and late diagnosis and initiation of treatment. Furthermore, HSCT for children is recently available in the medical center studied, but it was not accessible for some previous cases. The majority of the children with HLH died averagely within eight months post-diagnosis, and the mean length of follow-up in the treated patients was four years. The correlation of risk factors such as age at onset, gender, etiology, time to diagnosis, and central nervous system involvement with mortality was assessed in the current study. No correlation was found between the risk factors and mortality rate, which might be due to the limited number of patients investigated in the current study.
The current study had three limitations: first, genetic testing was not available in the hospital studied; therefore, the diagnosis was made based on other HLH-2004 Diagnostic Criteria. Second, for the classification of HLH as the familial form, genetic tests are necessary, while these tests were not available in the hospital studied; therefore, the cases with a sibling diagnosed with HLH were classified as familial cases. Third, the sample size was small in the current study; consequently, the correlation between some variables and mortality was not significant. It is recommend that future studies with larger sample sizes and genetic confirmation of the diagnosis be conducted.
5.1. Conclusions
HLH as a fatal disorder can develop at any age depending on the severity of the underlying genetic defect. Regarding the high risk of this disorder, recognition of the clinical features of HLH, especially atypical presentation of the disorder is critical both for pediatricians and subspecialists. Therefore, awareness improvement, timely identification and evaluation of the signs, and accurate management can result in favorable outcomes in patients with HLH.