1. Background
Diarrhea is still a major health problem in the world, especially in developing countries (1). Despite the reduction in mortality, diarrhea yet constitutes approximately 10% of deaths in children less than 5 years old and also causes about 500,000 deaths in developing countries per year (2, 3). Although viral agents such as rotavirus are the most common causes of acute diarrhea (lasting less than two weeks), bacterial agents are the prevalent reasons for acute diarrhea in developing countries (4). Bacterial diarrhea accounts for 34.5% - 60.7% of all diarrheas in developing countries and Shigella and enteropathogenic Escherichia coli are the main common causes (5, 6). The lack of access to clean water, the failure to observe personal hygiene (such as washing the hands), and lack of breastfeeding are the most predisposing factors for acute bacterial diarrhea (7, 8).
According to the high occurrence of vitamin D deficiency in children, researchers encounter the question of whether this vitamin has a role in the pathogenesis of acute bacterial diarrhea and whether vitamin D deficiency is a risk factor for bacterial diarrhea. Several studies have demonstrated the relationship between vitamin D deficiency and certain infectious diseases such as lower respiratory tract infections (9, 10). Vitamin D is a group of fat-soluble secosteroids. It has anti-bacterial and immunological effects in addition to playing a key role in bone metabolism. This vitamin is supplied through skin exposure to the sunlight or the consumption of foods rich in vitamin D such as fish oil (11, 12).
2. Objectives
Given the importance of understanding the factors contributing to infectious diarrhea and prevention of serious complications, the present study was performed to determine the association between serum 25 (OH) Vit. D level and acute bacterial diarrhea in children.
3. Methods
This study compared 60 children with bacterial diarrhea (the case group) and 60 healthy children (the control group) regarding the serum 25 (OH) Vit. D level. The study was conducted at Qazvin Children Hospital affiliated to Qazvin University of Medical Sciences (Qazvin, Iran). The age of children was between 2 months and 12 years. The inclusion criteria for the children affected with acute bacterial diarrhea were: (1) the presence of acute diarrhea (loose and watery stool more than three times/day for less than 14 days); (2) the presence of clinical symptoms such as fever, mucoid or bloody diarrhea, vomiting, and abdominal pain; (3) the presence of more than five white blood cells per microscopic field with or without red blood cells in stool examination; (4) the growth of bacteria in the cultured stool samples (4, 13, 14). Children with normal stool culture, underlying diseases or comorbidities (such as malnutrition, diabetes, kidney and liver disease, chronic diarrhea, etc.) and moreover, a history of previous antibiotic consumption and vitamin D supplements were excluded. Moreover, the children under 1 year with loose diarrhea without any dehydration and without any signs and symptoms of acute gastroenteritis (such as vomiting and abdominal pain) with normal stool examination and normal stool culture were excluded.
The control group was chosen by group matching of healthy children with no history of diarrhea in the past 3 months who visited in hospital’s health center for control routine or were hospitalized in the surgical department for elective surgery such as inguinal hernia. The two groups were matched regarding variables such as age, gender, breastfeeding (at least six months), family size, income, etc. (15, 16). Both groups lived in Qazvin province and were monitored by their local health centers until 2 years of age. Consecutive sampling continued until the desired sample size was completed. After approval of the project by Ethics Committee of the Qazvin University of Medical Sciences, written informed consent was obtained from children’s parents, and 3 mL of blood was collected to measure serum 25 (OH) Vit. D level. The samples were centrifuged and their sera were separated and kept at -20ºC. Then 25 (OH) Vit. D test was performed using ELISA method with a Euroimmun kit, Medizinische Labordiagnostika AG, Lübeck, Germany, EQ 6411-9601. Based on serum 25 (OH) Vit. D level, the children were divided into four groups: less than 10 ng/mL (severe vitamin D deficiency), 10 - 20 ng/mL (vitamin D deficiency), 20 - 30 ng/mL (suboptimal vitamin D level), and 30 - 50 ng/mL (optimal vitamin D level) (17). After collecting the fresh stool sample and placing it in a container of stool, it was immediately transferred to the laboratory department of the hospital. The stool examination and cultures were done by standard methods at Qazvin children hospital (18). The results were presented in statistical tables and numerical indicators. The data were analyzed with SPSS (version 16) using the chi-square test, Mann-Whitney’s U-test, and the t-test. The P < 0.05 was considered statistically significant.
3.1. Ethical Consideration
The Ethics Committee of the Qazvin University of Medical University confirmed the study (project No.: 297). All parents were completely explained before conducting the experiments. The children were included in the study after their parents’ permission and signed the written informed consent before the study.
4. Results
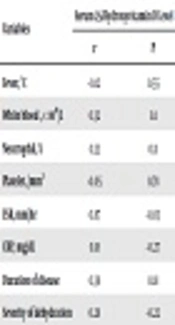
Among 60 patients with acute bacterial diarrhea, 38 (63.3%) were males and 22 (36.7%) females. In the control group, 31 (51.6%) were males and 29 (48.4%) females (P = 0.26). The minimum, maximum, and median (IQR) of age in the case group were 2 months, 5 years, and 16 (29) months, respectively. These values in the control group were 2months, 8 years, and 16 (24) months, respectively (P = 0.75). No significant difference was observed between the two groups regarding background and confounding variables (P > 0.05) (Table 1). The mean ± SD of 25 (OH) Vit. D concentrations in the case and control groups were 19.3 ± 7. 8 and 22.4 ± 7.3 ng/mL, respectively. There was a significant difference between the case and control groups regarding the serum 25-hydroxyvitamin D level (Table 2) (P = 0.02). There was no significant difference between the case and control groups in terms of severity of 25-hydroxyvitamin D deficiency (Table 3) (P = 0.16). There was no correlation between serum 25 (OH) Vit. D level with inflammatory and non-inflammatory markers (P > 0.05) (Table 4). The most common reasons for bacterial diarrhea in the present study were E. coli (n = 36, 60%), Shigella (n = 21, 35%), and Salmonella (n = 3, 5%). The enteropathogenic E. coli serotype and the sonnei serotype of Shigella were the most common pathogens.
| Variables | Case Group | Control Group | P Value |
|---|---|---|---|
| Sex, male/femalea | 38/22 | 31/29 | 0.26 |
| Age, mob | 16 (29) | 16 (24) | 0.75 |
| Weight, kgb | 10 (5.5) | 10 (5.6) | 0.82 |
| Height, cmb | 77.5 (23.7) | 78 (29.2) | 0.16 |
| Body mass indexb | 15.9 (3.1) | 17.1 (3.6) | 0.054 |
| Head circumference, cmb | 45 (7) | 45 (5) | 0.7 |
| Breast feeding, monthb | 14 ± 15.5 | 15 ± 16 | 0.61 |
| Family sizec | 3.9 ± 1.1 | 3.6 ± 0.8 | 0.26 |
| Income, Tomamc | 436 ± 137 | 464 ± 146 | 0.15 |
Comparison of Variables Between the Case and Control Groups
| Serum 25-Hydroxyvitamin D Level, ng/mL | Case Group, N = 60 | Control Group, N = 60 | P Value |
|---|---|---|---|
| Minimum | 6.5 | 9.1 | 0.02 |
| Maximum | 42.2 | 38.9 | |
| Mean ± SD | 19.3 ± 7. 8 | 22.4 ± 7.3 |
Comparison of Serum 25-Hydroxyvitamin D Level Between the Case and Control Groupsa
Severity of Serum 25-Hydroxyvitamin D Level in the Case and Control Groups
| Variables | Serum 25-Hydroxyvitamin D Level | |
|---|---|---|
| r | P | |
| Fever, °C | -0.12 | 0.55 |
| White blood ,× 109/L | 0.32 | 0.1 |
| Neutrophil, % | 0.33 | 0.11 |
| Platelet, /mm3 | -0.05 | 0.78 |
| ESR, mm/h | 0.87 | -0.03 |
| CRP, mg/dL | 0.18 | -0.27 |
| Duration of disease | 0.38 | 0.18 |
| Severity of dehydration | 0.28 | -0.22 |
5. Discussion
This study showed a significant relationship between serum 25 (OH) Vit. D concentrations and acute bacterial diarrhea. Studies in this area are contradictory (19-28). Bacterial agents are important causes of diarrhea in developing countries (1-4). Agents such as Shigella, pathogenic E. coli are the main common causes (5, 6). In the present study enteropathogenic E. coli, Shigella sonnei, and Salmonella were the most common pathogens. Although some studies suggested that vitamin D deficiency is a risk factor for diarrhea (19-25), others did not agree with this theory (27, 28). A study by Thornton et al. on 475 school-aged children with a mean age of 8.9 ± 1.6 years showed that 10% of the children suffered from vitamin D deficiency and 47% were vitamin D-insufficient. The one-year follow-up showed that the incidence of diarrhea is higher in children with vitamin D deficiency (19). Bener et al. study on 458 Qatari children showed that 61.6% of the children aged 11 - 16, 28.9% of the children 5 - 10 years old and furthermore, 9.5% of the children less than 5 years were vitamin D deficient. They reported that the incidence of diarrhea is significantly higher in children with vitamin deficiency (20). Additional studies have also confirmed the role of vitamin D deficiency in the prolongation and exacerbation of diarrhea caused by Clostridium difficile (6.1 days vs. 4.2 days, P = 0.01) (21, 22). Talachian et al. study on 25 children with acute diarrhea and 25 healthy children showed that serum 25 (OH) Vit. D concentrations are significantly lower in children affected with diarrhea (23). The study by Bucak et al. on 70 patients with rotavirus diarrhea and 60 healthy children as control group revealed that serum levels of vitamin D are significantly lower in children with diarrhea compared with healthy children (14.6 ± 8.7 ng/mL vs. 29.06 ± 6.51 ng/mL). These mentioned studies confirmed that vitamin D deficiency is a predisposing factor for rotavirus diarrhea (24).
Contrary to the mentioned reports, some studies believe that there is no relationship between vitamin D deficiency and diarrhea (26, 27). Urashima et al. study showed that vitamin D supplementation does not reduce the incidence of diarrhea (26). In addition, Ahmed et al. study on children below two years of old showed that normal-weight and underweight children with vitamin D deficiency less commonly develop diarrhea caused by EPEC, ETEC, and EAEC (27). However, the researchers could not justify the protective effect of vitamin D deficiency against these organisms and argued that this property may be specific to organisms that produce enterotoxin (27).
The difference in the results of mentioned studies may be related to many factors such as nutritional status, how much exposure to the sunlight, and the sample size. The results of the present study are consistent with studies that confirm the relationship between vitamin D and acute diarrhea. Although it is reported that vitamin D status can change during acute inflammation (25), we could not find any correlation between 25-hydroxyvitamin D and inflammatory markers such as fever, CRP, etc. These disparate findings in mentioned studies may be attributed to different factors such as age, type of pathogen responsible for diarrhea, laboratory definitions, type of sampling, and socioeconomic settings of studies.
Vitamin D is also produced by ultraviolet B radiation to the skin (28). Due to the abundance and stability of 25-hydroxyvitamin D in the blood, this form of metabolite is accepted to be used as an indicator of vitamin D deficiency in human (28). In addition to calcium control; vitamin D also has other functions such as immunomodulatory adjustment and anti-inflammatory and anti-bacterial properties (12). The antimicrobial role of vitamin D is due to the manufacture of antibacterial peptides such as cathelicidin and β-defensin in the epithelium of the digestive system as well as increased macrophage activity (12). These defense mechanisms increase the resistance against the invasion of intestinal pathogenic organisms such as Shigella and Salmonella (12, 20, 29). In cases of vitamin D deficiency, these defense mechanisms are destroyed and the risk of diarrhea is increased (30, 31). Animal investigations have shown that VDR expression is linked with the reduction of Salmonella invasion and furthermore, vitamin D-regulated antimicrobial peptides have anti-bactericidal effects on E. coli (32-34). Bucak et al. believe that vitamin D deficiency predisposes the human to rotavirus diarrhea (24).
The limitations of this study were: (1) lack of measurement of serum levels of vitamin D in patients with viral diarrhea due to the lack of virology testing and (2) the failure to re-measure the serum levels of vitamin D after recovery from diarrhea. Further studies are recommended in this area of research.
5.1. Conclusions
The present study revealed a significant correlation between serum 25 (OH) Vit. D level and bacterial diarrhea. It is likely that vitamin D plays a role in the pathogenesis of diarrhea.