1. Background
Nowadays, antibiotic-associated diarrhea (AAD) in children is of great concern due to the wide range of antibiotic administration among this population. Five to thirty-nine percent of children are under antibiotic therapy experience AAD (1). This rate even increases to 60% during hospital outbreaks (2). Clinical manifestation of this condition can vary from a loose-formed stool, urgency and abdominal cramp, infrequent painful, unfavorable conditions such as severe dehydration, electrolyte disturbances, and even the most severe symptom known as Clostridium difficile-associated diarrhea and toxic megacolon (3).
Numerous risk factors including age, poor hygiene, long-term hospitalization and particularly, prescription of broad-spectrum antibiotics have been considered reasons for AAD incidence (4, 5). The occurrence of AAD is high in certain conditions, including the use of oral antibiotics, antibiotics against anaerobic bacteria, and using some antibiotics such as clindamycin, cephalosporins, broad-spectrum penicillins, and Amoxicillin-clavulanate (6, 7).
Probiotics are defined as non-pathogenic living microorganisms which in case of adequate and timely administration, provide benefits for the hosts. Galacto-oligosaccharides and fructooligosaccharides, known as prebiotics, are compounds that are added to probiotics to induce the growth or activity of them. The combination of the mentioned compounds is defined as synbiotics (8).
Numerous studies have presented the advantages of probiotic use for diarrhea treatment (9, 10). Furthermore, this material has been successfully tested for irritable bowel syndrome, inflammatory bowel disease, atopic dermatitis and other allergic conditions (11-15).
Various studies presented that the combination of probiotics was associated with better rehabilitation in patients resenting from diarrhea. On the other hand, studies regarding the use of synbiotic in children and in the prevention and treatment of AAD are rare and have controversial outcomes (16, 17). Given this purpose, this study was conducted to assess the effectiveness of synbiotics on AAD in children.
2. Methods
This randomized, double-blinded, placebo-controlled trial was performed on 100 patients admitted to Imam Hossein Hospital (affiliated with Isfahan University of Medical Sciences) in 2017 - 2018.
All of the 2 months to 14-year-old children admitted to Imam Hossein Hospital who required antibiotic therapy for over five days, and their parents who were willing to participate in this study were included. Children with the following conditions were not studied in the research; presence of acute or chronic diarrhea initiated prior to the current antibiotic therapy, a history of antibiotic therapy in recent two months, use of prophylactic antibiotics, a history of Clostridium difficile-associated diarrhea in previous three months, any history of underlying gastrointestinal disorders, the use of probiotics during previous seven days, immunodeficiency and long-term administration of drugs affecting gastrointestinal tract. Patients who had any of the following measures were excluded from the study: child’s or parents’ unwillingness for participation or continuing study protocol, the occurrence of any possible serious side effects due to synbiotic therapy and less than 70 percent of adherence to study protocol.
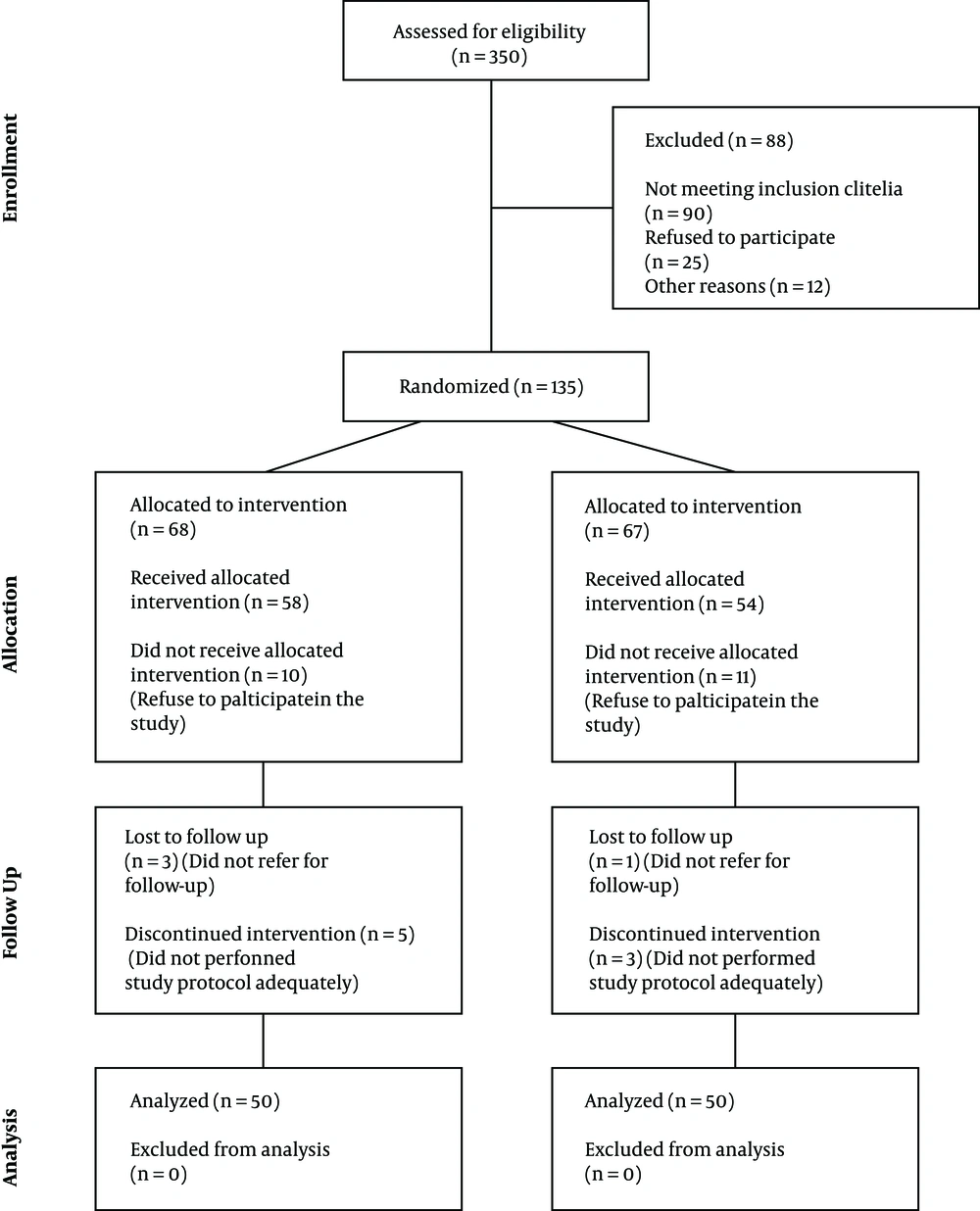
Figure 1 Represents consort diagram of the study.
Following the approval of the study protocol by Isfahan University of Medical Sciences Ethics Committee (code: IRCT20171119037543N2), all information about the research process was explained for children and their parents in detail and parents signed written consent for the participation of their children in the study.
One-hundred patients meeting the criteria were included in the study by convenience sampling. They were randomly divided into the two subgroups of the case group (treated with synbiotic) and control (treated with placebo).
Patients and their parents were blinded to the therapy they received. This blindness was also performed for the physician who assessed the diarrhea status of participants.
Patients in the case and control groups received their remedies within 24 hours after antibiotic therapy initiation at most. The case group was treated with synbiotics which contained CFU109 colony count, containing Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Lactobacilus acidophilus, Bifidobacterium breve, Bifidobacterium infantis and Lactobacillus bulgaricus (Protexin; The United Kingdom) and the control group was treated with the sachets similar in color, size, and shape with synbiotics produced by the same company. Mentioned remedies were daily used up to 7 days after antibiotic therapy cessation, according to medication instructions provided by the manufacturer.
To check patients’ adherence to the treatment protocol, the parents were inquired and the number of sachets consumed by the patient during the treatment period was counted. This checking was done with the following frequencies: every day during hospitalization, every three days before antibiotic therapy cessation, and every two days until the end of the interventions.
In the case of diarrhea incidence, patients’ stool exam was assessed regarding the presence of occult or overt blood, white blood cells, and mucus. Antibiotic-associated diarrhea was defined as the incidence of diarrhea due to antibiotic use from its initiation to three weeks after antibiotic therapy cessation, with or without synbiotics consumption. The determination of stool consistency was done based on the Bristol Stool Scale (BSS) (18). Accordingly, defecation ≥ 3 times a day for ≥ 2 days with the stool consistency of ≥ 6 based on BSS was defined as severe diarrhea and defecation ≥ 2 times a day for ≥ 2 days with the stool consistency of ≥ 5 based on BSS was defined as mild diarrhea (19). Any change in the stool consistency to an upper grade of BSS before antibiotic therapy remaining for at least 48 hours was considered loose stool.
The following research data were recorded on a checklist: demographics (age and gender), primary and final diagnosis given by a pediatric infection specialist, prescribed antibiotics, the number of defecations and consistency of stool (prior to antibiotic therapy and after AAD), duration of diarrhea, the presence of blood or mucus in stool and other complications (including fever, vomiting, abdominal pain, constipation, flu and flu-like symptoms, irritability, and drowsiness).
The collected data were analyzed using SPSS V. 22 software (IBM SPSS®; The United States). Descriptive data were presented in mean and percentages. Analytic data were analyzed using covariance analysis, chi-square test, and logistic regression test. The P value of less than 0.05 was considered significant.
3. Results
In the current study, 100 patients requiring antibiotic therapy were assessed, fifty of whom were considered the case group, and the other half was regarded as the control group. The mean age of participants was 4.40 ± 3.47 years. Fifty-seven patients were males, and forty-three were females. Forty-nine patients developed AAD. The mean duration of patients’ hospitalization was 9.36 ± 8.70 days, defecation frequency before antibiotic therapy was 1.61 ± 0.75, AAD occurred on day 5.91 ± 6.43 after the start of antibiotics, and the duration of diarrhea was 8.7 ± 6.64 days. Table 1 presents further information about the study and control groups in detail. As it is shown in this the the frequency of AAD occurrence in the case group was significantly less than the control group (P = 0.016), while other variables were not statistically different between the two studied groups (P > 0.05).
| Variable | Total (N = 100) | Case Group (N = 50) | Control Group (N = 50) | P Value |
|---|---|---|---|---|
| Age (y) | 4.40 ± 3.47 | 4.64 ± 3.82 | 4.18 ± 3.11 | 0.51 |
| Gender | 0.21 | |||
| Male | 57 (57) | 31 (62) | 26 (52) | |
| Female | 43 (43) | 19 (38) | 24 (48) | |
| Length of hospitalization | 9.36 ± 8.70 | 8.21 ± 3.67 | 10.5 ± 11.80 | 0.17 |
| Frequency of defecation prior to antibiotic therapy | 1.60 ± 0.76 | 1.68 ± 0.85 | 1.51 ± 0.63 | 0.28 |
| Stool type prior to synbiotic/placebo treatment based on the Bristol Stool Scale | 3.60 ± 0.09 | 3.76 ± 1 | 3.60 ± 0.80 | 0.38 |
| Antibiotic-associated diarrhea occurrence | 49 (49) | 18 (36) | 31 (62) | 0.016 |
| Duration of antibiotic-associated diarrhea | 8.70 ± 6.64 | 8.10 ± 6.50 | 9.03 ± 6.81 | 0.51 |
| Frequency of defecation following synbiotic/placebo treatment | 1.50 ± 0.53 | 1.46 ± 0.47 | 1.57 ± 0.58 | 0.18 |
| Stool type following synbiotic/placebo treatment based on the Bristol Stool Scale | 3.70 ± 0.70 | 3.70 ± 0.71 | 3.85 ± 0.70 | 0.26 |
aValues are expressed as mean ± SD or No. (%).
Table 2 demonstrates the primary diagnosis and final diagnosis of hospitalized patients who received antibiotic therapy.
| Type of disease | Primary Diagnosis | Final Diagnosis | ||
|---|---|---|---|---|
| Control Group | Case Group | Control Group | Case Group | |
| Staphylococcal scalded skin syndrome | 1 (3.2) | 1 (5.6) | 1 (3.2) | 1 (5.6) |
| Abscess | 6 (19.4) | 2 (11.1) | 4 (12.9) | 3 (16.7) |
| Arthritis | 4 (12.9) | 2 (11.1) | 3 (9.7) | 1 (5.6) |
| Cellulitis | 7 (22.6) | 4 (22.2) | 6 (19.4) | 4 (22.2) |
| Central nervous system infection | 3 (9.7) | 2 (11.1) | 2 (6.5) | 2 (11.1) |
| Lymphadenitis | 5 (16.1) | 5 (27.8) | 4 (12.9) | 3 (16.7) |
| Mastoiditis | 1 (3.2) | 1 (5.6) | 0 (0) | 1 (5.6) |
| Sepsis | 0 (0) | 1 (5.6) | 1 (3.2) | 1 (5.6) |
| Sinusitis | 1 (3.2) | 0 (0) | 1 (3.2) | 0 (0) |
| Urinary tract infection | 2 (6.5) | 0 (0) | 2 (6.5) | 0 (0) |
| Other diagnoses | 1 (3.2) | 0 (0) | 7 (22.6) | 2 (11.1) |
| P value | 0.845 | 0.863 | ||
aValues are expressed as No. (%).
Table 3 represents antibiotics prescribed for the patients. Considering the type of antibiotic therapy, no significant difference was detected between the case group and controls regarding AAD incidence (P = 0.438). Although the duration of antibiotic therapy was 12.98 ± 5.84 days in the control group experiencing AAD, it was 15.55 ± 6.95 days in the case group. Nevertheless, no statistical difference was found between the two groups regarding the duration of antibiotic therapy (P = 0.17).
| Type of Antibiotic/Antibiotics’ combination | Control Group | Case Group | Total | |||
|---|---|---|---|---|---|---|
| Total | ADD | Total | AAD | Total | ADD | |
| Amoxicillin_Ceftriaxone | 2 (4.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 1 (1.6) |
| Azithromycin_Amoxicillin | 1 (2.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.6) |
| Cefixime_Amoxicillin | 1 (2.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.6) |
| Cefixime_Cephalexin_Clindamycin | 1 (2.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.6) |
| Cefotaxime_Ampicillin | 1 (2.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.6) |
| Cefotaxime_Vancomycin | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1 (5.6) | 1 (1.0) | 1 (2.8) |
| Ceftriaxone | 5 (10.0) | 2 (6.5) | 2 (4.0) | 0 (0.0) | 7 (7.0) | 2 (3.3) |
| Ceftriaxone_Cefixime | 1 (2.0) | 0 (0.0) | 1 (1.0) | |||
| Ceftriaxone_Clindamycin_Cephalexin | 1 (2.0) | 0 (0.0) | 1 (2.0) | 1 (5.6) | 2 (2.0) | 1 (2.8) |
| Ceftriaxone_Vancomycin | 0 (0.0) | 0 (0.0) | 3 (6.0) | 1 (5.6) | 3 (3.0) | 1 (2.8) |
| Ceftriaxone_Vancomycin_Amoxicillin | 1 (2.0) | 1 (2.0) | 2 (2.0) | |||
| Ceftriaxone_Vancomycin_Cephalexin | 0 (0.0) | 1 (2.0) | 1 (1.0) | |||
| Ceftriaxone_Vancomycin_Metronidazole | 2 (4.0) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 2 (3.3) |
| Ceftriaxone-Amoxicillin | 1 (2.0) | 0 (0.0) | 1 (1.0) | |||
| Cephalexin | 2 (4.0) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 2 (3.3) |
| Cephalexin_Ampicillin | 0 (0.0) | 1 (2.0) | 1 (1.0) | |||
| Cephalexin_Vancomycin_Ceftriaxone | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1 (5.6) | 1 (1.0) | 1 (2.8) |
| Clindamycin | 13 (26.0) | 10 (32.3) | 9 (18.0) | 3 (16.7) | 22 (22.0) | 13 (24.5) |
| Clindamycin_Amoxicillin | 2 (4.0) | 1 (3.2) | 2 (4.0) | 1 (5.6) | 4 (4.0) | 2 (4.4) |
| Clindamycin_Cefixime | 1 (2.0) | 1 (3.2) | 1 (2.0) | 0 (0.0) | 2 (2.0) | 1 (1.6) |
| Clindamycin_Cefixime_Ceftriaxone | 1 (2.0) | 1 (3.2) | 3 (6.0) | 1 (5.6) | 4 (4.0) | 2 (4.4) |
| Clindamycin_Cefixime_Ceftriaxone_Metronidazole | 0 (0.0) | 1 (2.0) | 1 (1.0) | |||
| Clindamycin_Cefixime_Cephalexin | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1 (5.6) | 1 (1.0) | 1 (2.8) |
| Clindamycin_Ceftriaxone | 3 (6.0) | 1 (3.2) | 2 (4.0) | 1 (5.6) | 5 (5.0) | 2 (4.4) |
| Clindamycin_Ceftriaxone_Amoxicillin | 0 (0.0) | 2 (4.0) | 2 (2.0) | |||
| Clindamycin_Ceftriaxone_Cephalexin | 2 (4.0) | 0 (0.0) | 2 (2.0) | |||
| Clindamycin_Ceftriaxone_Metronidazole | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1 (5.6) | 1 (1.0) | 1 (2.8) |
| Clindamycin_Cephalexin | 6 (12.0) | 5 (16.1) | 9 (18.0) | 3 (16.7) | 15 (15.0) | 8 (16.4) |
| Clindamycin_Vancomycin | 1 (2.0) | 0 (0.0) | 1 (2.0) | 1 (5.6) | 2 (2.0) | 1 (2.8) |
| Clindamycin_Vancomycin_Ceftriaxone | 0 (0.0) | 0 (0.0) | 1 (2.0) | 1 (5.6) | 1 (1.0) | 1 (2.8) |
| Vancomycin | 1 (2.0) | 1 (2.0) | 2 (2.0) | |||
| Vancomycin_Ceftriaxone | 1 (2.0) | 1 (3.2) | 4 (8.0) | 1 (5.6) | 5 (5.0) | 2 (4.4) |
| Vancomycin_Ceftriaxone_Cefixime | 0 (0.0) | 1 (2.0) | 1 (1.0) | |||
| Total | 50 (100.0) | 31 (100.0) | 50 (100.0) | 18 (100.0) | 100 (100.0) | 49 (100.0) |
| P Value | 0.38 | 0.43 | ||||
aValues are expressed as No. (%)
Logistic regression test showed that the control group was significantly at a higher risk of AAD (OR = 2.4; 95% CI: 1.09 - 5.46; P = 0.02).
4. Discussion
Antibiotics are the most common therapeutic agents utilized widely for the treatment of hospitalized children. Antibiotic-associated diarrhea and its related adverse effects, i.e., Clostridium difficile-associated diarrhea as the most serious complication in this vulnerable population are of great concern for pediatricians (20).
Physicians of ancient Persia recommended salty yogurt for the rehabilitation of intestinal diseases, appetite stimulation, and diarrhea improvement (21). There are studies in this regard that have shown probiotic yogurt can successfully affect viral diarrhea and shorten its duration; therefore, it shortens the duration of the hospitalization (22).
There are many studies evaluating the effect of probiotics on AAD (3, 20) most of which have declared significant advantages of probiotic use for both prevention and earlier rehabilitation of patients resenting from AAD (23-26), while some other studies have declared the lack of any benefit (27, 28). However, regarding the effectiveness of synbiotic on AAD, the number of studies is limited (29).
In the current study, we assessed the effectiveness of synbiotics on AAD in children under antibiotic treatment. The case and control groups were not statistically different regarding age, gender distribution, length of hospitalization, the frequency of defecation and stool consistency based on BSS before antibiotic therapy. The primary and final diagnosis of children which caused them to be hospitalized, the type of antibiotics prescribed for them, and the duration of antibiotic therapy were not statistically different as well. Eliminating the effects of the above confounding variables, the results of our study could be attributed only to the effect of synbiotics.
The current study has shown that the use of synbiotics within 24 hours following the antibiotic therapy initiation caused a statistically fewer occurrence of AAD in the case group compared with the control group, and the logistic regression test shows that children who were not under synbiotic therapy were at 2.4 times higher risk of AAD in comparison to the case group. On the other hand, in the case of occurrence of diarrhea, synbiotics use cannot significantly affect the duration of diarrhea, consistency of patients’ stool based on BSS, and times of defecation a day.
A similar case-control study conducted by Jafari et al., which showed no benefit of synbiotics prescription for children under antibiotic therapy. They declared that the use of synbiotics could not prevent antibiotic-associated diarrhea in children who received antibiotics. Furthermore, children with AAD who received synbiotic treatment did not present superior outcomes regarding the duration of diarrhea, stool consistency, and even times of defecation a day. Although their findings of diarrhea prevention were inconsistent with ours, their conclusion about other mentioned variables confirmed our results. This difference may be due to a shorter duration of synbiotics administration as they prescribed synbiotic only for a week, while we performed our study from initiation of antibiotic therapy up to 7 days after its cessation (16).
The other study by Dinleyici et al. applied synbiotics to hospitalized children due to acute diarrhea regardless of the etiology of diarrhea. They found that the use of synbiotics was accompanied by a reduction of at least a day in hospitalization and a mean reduction of 36 hours of diarrhea. The duration of synbiotic treatment was only five days in their study, and as they used synbiotic after diarrhea initiation, they did not present any data about the ability of synbiotics in diarrhea prevention (30).
Passariello et al. in the study conducted in 2012, assessed the effectiveness of new synbiotics consisted of Lactobacillus paracasei B21060, arabinogalactan and xylooligosaccharides. They performed their study on children complaining of diarrhea regardless of its etiology and concluded that the use of synbiotic could significantly reduce the duration of diarrhea, times of defecation a day, and improve stool consistency (31).
Vandenplas et al. conducted their study on children with acute infectious diarrhea to assess the cost-effectiveness of synbiotics prescription. They concluded that the use of synbiotic was accompanied by 24 hours earlier improvement in diarrhea and reduces 25% of the overall hospital costs by decreasing the length of stay, add-on therapy, and further consultation (32).
Further evaluation represented that although the use of probiotics and synbiotics may not statistically affect the duration of ADD, the frequency of defecation, stool consistency, and their efficacy remains to be clarified, a decrease in hospitalization duration may be the merits of their use (33-35). The strengths of the present study are the use of valid criteria for the diagnosis and the assessment of the severity of diarrhea, i.e., BSS. This study is also among the few studies that evaluated the effect of synbiotics on ADD and in the pediatric age group. We evaluated the effect of synbiotics in a wide range of infectious diseases, and also different prescribed antibiotics alone and combined. The effectiveness of synbiotics in the prevention and improvement of ADD was adequately addressed in this study as it was used at the initiation of antibiotics and continued for a week after cessation of antibiotic therapy.
4.1. Conclusions
In summary, the findings of this study showed that early initiation of synbiotics and its long-term administration following antibiotic therapy cessation could considerably prevent antibiotic-associated diarrhea incidence. However, synbiotics use could not positively affect the duration, stool consistency, and frequency of defecation a day in AAD-affected patients.