1. Background
Scrub typhus is a common bacterial zoonosis caused by a Gram-negative bacterium known as Orientia tsutsugamushi. This infection is transmitted to humans from the bite of a trombiculid mite (1). It was originally found in the Himalayan foothills and Southeast Asia, whereas it has now spread to different parts of India, with higher incidence rates reported in southern and northeastern regions of the country. The tsutsugamushi triangle, which comprises of Southeast Asia, Asian Pacific Rim, and Northern Australia, accounts for the largest burden of the disease (1, 2). Although it was considered to be a rare disease for decades and practically obsolete after the Second World War (3), there has been a clear re-emergence of the disease in many parts of India and Burma.
The clinical manifestations of scrub typhus are attributed to acute vasculopathy that ensues when the organism attaches to and penetrates into phagocytes and endothelial cells by a specific 56-KDA protein. Fever, headache, drowsiness, conjunctival suffusion, lymphadenopathy, and hepatosplenomegaly are the common clinical manifestations of scrub typhus. A characteristic eschar can be seen at variable rates (10% - 92%) at the site of the chigger bite (4). Severe complications include septic shock, acute kidney injury, meningoencephalitis, myocarditis, disseminated intravascular coagulation (DIC), and multi-organ dysfunction syndrome, which may occur in a variable number of patients.
It is known that diagnostic tests for scrub typhus, such as Weil-Felix test, lack sensitivity and specificity. In India, the current serological diagnostic test continues to be immunoglobulin M enzyme-linked immunosorbent assay (IgM ELISA), as the gold standard immunofluorescence antibody (IFA) kit is not readily available in the country. Although the majority of previous studies have emphasized on the clinical aspects of this disease, there are insufficient studies on markers of its severity (5). Therefore, it is important to study the markers of disease severity, which can predict the clinical course and complications and facilitate early treatment.
2. Objectives
In this retrospective study, we aimed to present a detailed clinical profile of scrub typhus and identify the predictors of its severity.
3. Methods
This retrospective study was conducted at Sri Ramachandra Medical College and Research Institute, Chennai, India from January 2015 to November 2017. Children who were clinically symptomatic and positive for scrub typhus IgM were included in the analysis. Data were collected from a detailed proforma, which contained clinical details and laboratory reports of patients, including complete blood cell count, liver function tests, kidney function tests, serum electrolytes, routine urine tests, scrub typhus IgM, and other relevant examinations.
Scrub typhus was diagnosed based on major clinical symptoms including acute fever and at least one of the minor symptoms, such as myalgia, headache, conjunctival injection, cough, profuse sweating, maculopapular rash, lymphadenopathy, eschar, and positive IgM ELISA results (6). The exclusion criteria in this study were as follows: children using long-term medications, immunodeficiency, and have a chronic disease. Severity of the disease was defined based on previously published adult and pediatric studies (7-13). The study population was divided into two groups in terms of disease severity: (1) non-severe, patients without any complications and (2) severe, patients with severe complications. Out of 235 patients who tested positive for scrub typhus, 39 were classified in the severe group and 196 in the non-severe group.
3.1. Definition of Severe Scrub Typhus
Severe scrub typhus was diagnosed in patients with a clinical image of organ dysfunction (Table 1).
| Involved Organs | Complications | Clinical Definition |
|---|---|---|
| Respiratory system | Acute respiratory distress syndrome | PaO2/FiO2 ratio ≤ 200 mmHg; bilateral infiltrates on a chest radiograph; no evidence of left-sided heart failure/cardiomegaly |
| Pneumonia | Clinical and radiological evidence of pneumonia | |
| Cardiovascular system | Myocarditis | Congestive heart failure/cardiomegaly; Inotrope requirement > 5 µg/kg/min (dopamine/dobutamine); left ventricular dysfunction based on the echocardiogram without a history of cardiomyopathy; elevated serum MB fraction of creatine phosphokinase; abnormal electrocardiography |
| Shock | Reduction of blood pressure (hypotension) < 5th percentile for age, sex, and height or systolic blood pressure < 2 SD below the normal range for age; fluid refractory shock requiring inotropes | |
| Blood parameters | Severe thrombocytopenia and bleeding tendency | Platelet count < 20,000/mm3; spontaneous bleeding tendency; increased prothrombin time |
| Abdominal system | Acute kidney injury | Absolute increase in serum creatinine ≥ 0.3 mg/dL (≥ 26.4 mol/L); percentage increase in serum creatinine ≥ 50% (1.5 fold from baseline); reduction of urine output (documented oliguria < 0.5 mL/kg per hour for more than six hours) |
| Hepatic dysfunction | More than a four-fold increase in liver transaminases with or without bilirubin > 2 mg/dL | |
| Central nervous system | Meningoencephalitis | Neurological symptoms including headache, altered sensorium, seizure, and vomiting with; meningeal signs, papilledema or; CSF analysis indicating pleocytosis and elevated protein level |
Clinical Definition of Severe Scrub Typhus in Children
3.2. Statistical Analysis
Categorical data are expressed as frequency. Differences between the two groups were analyzed using chi-square test. Multivariate logistic regression analysis and chi-square test were performed to determine the associations between variables and severity of disease for predicting the severity of scrub typhus. The final data are presented as unadjusted odds ratio (OR) at 95% confidence interval (CI). P value less than 0.05 was considered statistically significant. Statistical analyses were performed in SPSS version 23.0 (SPSS Inc., Chicago, Ill., USA).
4. Results
A total of 235 patients were included in this study. The study population was divided into two groups of severe and non-severe scrub typhus, based on the defined criteria. Clinical parameters were compared between the two groups (Table 2). According to multivariate regression analysis, some parameters were found to be strong predictors of the severity of the disease.
| Characteristics | Severe Scrub Typhus (N = 39) | Non-Severe Scrub Typhus (N = 196) | P Value |
|---|---|---|---|
| Age, y | 0.190 | ||
| < 5 | 19 (48.7) | 67 (34.2) | |
| 6 - 10 | 8 (20.5) | 42 (21.4) | |
| 11 - 18 | 12 (30.8) | 87 (44.4) | |
| Gender | 0.160 | ||
| Male | 16 (41) | 107 (54.6) | |
| Female | 23 (59) | 89 (45.4) | |
| PICU stay | 20 (51.3) | 20 (10.2) | < 0.001 |
| Fever | 38 (97.4) | 193 (98.5) | 0.519 |
| Myalgia | 2 (5.1) | 26 (13.3) | 0.185 |
| Eschar | 10 (25.6) | 42 (21.4) | 0.534 |
| Jaundice | 1 (2.6) | 3 (1.5) | 0.519 |
| Cough | 12 (30.8) | 59 (30.1) | 1.00 |
| Dyspnea | 4 (10.3) | 7 (3.6) | 0.089 |
| Oliguria | 6 (15.4) | 26 (13.3) | 0.798 |
| Vomiting | 13 (23.3) | 60 (30.6) | 0.710 |
| Diarrhea | 9 (23.1) | 17 (8.7) | 0.021 |
| Hepatomegaly | 3 (7.7) | 23 (11.7) | 0.585 |
| Splenomegaly | 4 (10.3) | 31 (15.8) | 0.467 |
| Altered sensorium | 4 (10.3) | 3 (1.5) | 0.016 |
| Abdominal pain | 11 (28.2) | 37 (18.9) | 0.196 |
| Seizure | 9 (23.1) | 0 (0) | < 0.001 |
Clinical Characteristics of Patients with Severe and Non-Severe Scrub Typhusa
Approximately 20% of children with scrub typhus below 10 years of age developed the severe form of the disease versus 12% of children in the age group of 10 - 18 years. Younger patients seem to be more susceptible to severe scrub typhus, compared to older children, as they are actively involved in recreational activities, associated with higher outdoor exposure.
The findings showed that more than 50% of patients in the severe scrub typhus group required PICU stay versus 10.2% of patients in the non-severe group. The most common reasons for PICU stay were hemodynamic instability and organ dysfunction. Only 20% of the patients developed eschar, and the difference between the two groups was not significant. Gastrointestinal (GI) involvement was reported in 23.1% of children with severe scrub typhus versus only 8.7% in the non-severe group, presenting with diarrhea.
Moreover, 33.4% of children with severe scrub typhus showed symptoms of central nervous system (CNS) involvement (e.g., altered sensorium and seizures) versus < 2% in the non-severe group.
Based on the findings, there was no significant difference between the two groups with regard to clinical parameters, such as sex, jaundice, cough, myalgia, dyspnea, oliguria, vomiting, hepatomegaly, splenomegaly, abdominal pain, headache, rash, and edema.
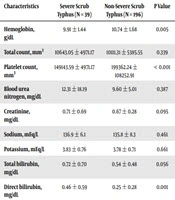
Comparison of laboratory parameters (Table 3) showed that the hemoglobin level was significantly different between the two groups (P < 0.005), and the severe scrub typhus group had lower hemoglobin levels. Comparison of platelet count showed a significant difference between the two groups (P < 0.001), although its clinical relevance was minimal. On average, platelet count in the severe scrub typhus group was lower than that of the non-severe group.
| Characteristics | Severe Scrub Typhus (N = 39) | Non-Severe Scrub Typhus (N = 196) | P Value |
|---|---|---|---|
| Hemoglobin, g/dL | 9.91 ± 1.44 | 10.74 ± 1.68 | 0.005 |
| Total count, mm3 | 10643.05 ± 4971.17 | 10111.31 ± 5385.55 | 0.339 |
| Platelet count, mm3 | 149143.59 ± 4971.17 | 199362.24 ± 108252.91 | < 0.001 |
| Blood urea nitrogen, mg/dL | 12.31 ± 18.19 | 9.60 ± 5.01 | 0.387 |
| Creatinine, mg/dL | 0.71 ± 0.69 | 0.67 ± 0.28 | 0.095 |
| Sodium, mEq/L | 136.9 ± 6.1 | 135.8 ± 8.3 | 0.461 |
| Potassium, mEq/L | 3.83 ± 0.76 | 3.78 ± 0.71 | 0.661 |
| Total bilirubin, mg/dL | 0.72 ± 0.70 | 0.54 ± 0.48 | 0.056 |
| Direct bilirubin, mg/dL | 0.46 ± 0.59 | 0.25 ± 0.28 | 0.001 |
| Serum glutamic oxaloacetic transaminase (SGOT), IU/L | 204.1 ± 143.1 | 65.1 ± 43.3 | < 0.001 |
| Serum glutamic pyruvic transaminase (SGPT), IU/L | 167 ± 145.6 | 50.8 ± 26.1 | < 0.001 |
| Serum albumin, g/dL | 3.07 ± 0.72 | 3.37 ± 0.62 | 0.007 |
Laboratory Findings of Patients with Severe and Non-Severe Scrub Typhusa
According to the liver function tests, liver enzymes (SGOT and SGPT) were found to be markedly higher in patients with severe scrub typhus. The average levels of SGOT and SGPT in the severe scrub typhus group were more than five times higher than the average SGOT and SGPT in the non-severe group. The bilirubin level, serum albumin, prothrombin time, and partial thromboplastin time were compared between the two groups, and the difference was statistically significant.
Multivariate regression analysis was conducted to compare the laboratory findings of the two groups (Table 4). Comparisons showed the deterioration of different parameters in the group with severe scrub typhus. Serum albumin was markedly lower in patients with severe scrub typhus. The number of patients with serum albumin levels below 2.5 g/dL was lower in the severe scrub typhus group, compared to the non-severe group (OR = 3.185; CI = 1.427 to 7.110; P = 0.006). The findings showed that the SGOT level was significantly higher in the severe scrub typhus group, compared to the non-severe group (OR = 49.7; CI = 13.490 to 183.179; P < 0.001). According to the results, despite the increased enzyme levels of patients with severe scrub typhus, none of them experienced hepatic failure.
| Characteristics | Severe Scrub Typhus (N = 39) | Non-Severe Scrub Typhus (N = 196) | OR | CI | P Value |
|---|---|---|---|---|---|
| PICU stay | 20 (51.3) | 20 (10.2) | 9.263 | 4.247 - 20.204 | < 0.001 |
| Diarrhea | 9 (23.1) | 17 (8.7) | 3.159 | 1.290 - 7.736 | 0.021 |
| Altered sensorium | 4 (10.3) | 3 (1.5) | 7.352 | 1.577 - 34.284 | 0.016 |
| Hemoglobin (< 10 g/dL) | 23 (59) | 66 (33.7) | 2.831 | 1.401 - 5.722 | 0.004 |
| Total cholesterol (> 10,000/mm3) | 20 (51.3) | 85 (43.4) | 1.375 | 0.690 - 2.737 | 0.383 |
| Platelet count (< 150,000/mm3) | 27 (69.2) | 92 (46.9) | 2.543 | 1.219 - 5.307 | 0.014 |
| Blood urea nitrogen (> 40 mg/dL) | 2 (5.1) | 1 (0.05) | 10.541 | 0.932 - 119.257 | 0.072 |
| Creatinine (> 2 mg/dL) | 1 (2.6) | 1 (0.5) | 5.132 | 0.314 - 83.84 | 0.305 |
| SGOT (> 200 IU/L) | 17 (43.6) | 33 (1.5) | 49.7 | 13.490 - 183.179 | < 0.001 |
| Albumin (< 2.5 g/dL) | 12 (30.8) | 24 (12.2) | 3.185 | 1.427 - 7.110 | 0.006 |
| Prothrombin time (> 15 s) | 22 (56.4) | 72 (36.7) | 2.229 | 1.111 - 4.472 | 0.031 |
| Partial thromboplastin time (> 40 s) | 6 (15.4) | 15 (7.7) | 2.194 | 0.794 - 6.065 | 0.130 |
Logistic Regression Analysis of Severe and Non-Severe Scrub Typhusa
Based on the results, the average platelet count was lower in the severe scrub typhus group, compared to the non-severe group. Comparison of the number of patients with thrombocytopenia (< 150,000 cells/cumm) between the two groups showed that most patients belonged to the severe scrub typhus group (OR = 2.543; CI = 1.219 to 5.307; P = 0.014). Moreover, comparison of patients with hemoglobin levels below 10 g/dL showed that most of these patients belonged to the severe scrub typhus group (OR = 2.831; CI = 1.401 to 5.722; P = 0.004).
According to the odds ratio measurements, other significant parameters included altered sensorium, blood urea nitrogen level, creatinine level, and PICU stay. These parameters were expected to be higher in patients with severe scrub typhus. In addition, comparison of clinical presentations showed that the incidence of diarrhea was higher among patients with severe scrub typhus (OR = 3.159; CI = 1.290 to 7.736; P = 0.02).
Table 5 presents the complications of patients with scrub typhus. The incidence of complications was reported in comparison with the total number of patients without the complication, to prevent any untoward bias related to patients with multiple complications. A total of 235 patients presented with scrub typhus, 38 of whom experienced difficulties. Shock was the most common complication, as reported in 16 patients (6.8% of the study population), followed by renal failure (2.1%) and seizure (2.1%). The mortality rate was 1.3% in our population, which is similar to the rate reported by Kim et al., (0.5%) (11).
| Complications | Absent | Present |
|---|---|---|
| Pneumonia | 233 (97.9) | 2 (0.9) |
| Renal failure | 230 (97.9) | 5 (2.1) |
| Coagulopathy | 233 (99.1) | 2 (0.9) |
| DIC | 233 (99.1) | 2 (0.9) |
| Shock | 219 (93.2) | 16 (6.8) |
| Myocarditis | 233 (99.1) | 1 (0.4) |
| Hepatic failure | 235 (100) | 0.0 |
| Meningoencephalitis | 231 (98.3) | 4 (1.7) |
| Seizure | 230 (97.9) | 5 (2.1) |
| Multiple organ dysfunction syndrome | 234 (99.6) | 1 (0.4) |
| Death | 232 (98.7) | 3 (1.3) |
Complications of Children with Scrub Typhusa
5. Discussion
In this study, we collected the data of 235 patients with scrub typhus over three years and analyzed the results. The proportion of patients with severe scrub typhus was 16.6%, which is lower than the rate reported by Kim et al. (11), (42.8%). On the other hand, two studies from India reported higher rates than our study. A study by Karanth et al. (14), from Karnataka, India, demonstrated that 52.7% of patients had severe scrub typhus, while another study from Pondicherry reported a prevalence of 40.6%. In the study by Karanth et al. (14), the number of patients with severe scrub typhus was higher in younger age groups, while the incidence of severe scrub typhus was lower in children above five years. The possible reason for this finding may be the robust immune system of older children, compared to the younger age groups.
In the present study, only 22.13% of the patients presented with eschar. There was no significance difference between the two groups with regard to eschar. In this regard, Karanth et al. (14), reported eschar in 40.38% of patients in the non-severe group. Conversely, in the study by Narayanasamy et al. (15), only 25% of patients with severe scrub typhus developed eschar. Kim et al. (11), reported that the absence of eschar is one of the predictors of disease severity in adults. Based on these findings, no clear evidence suggests that eschar is one of the predictors of disease severity. The causes of difference between these studies may be the location of the study populations, clinical knowledge of physicians about eschar, and earlier detection of eschar in febrile patients. The lower prevalence of eschar could be attributed to the dark skin color of the Indian population and the unusual feeding sites of chiggers (16, 17).
In the present study, the proportion of patients with GI symptoms was much higher in the severe group, whereas no such association was found in previous studies. Therefore, further studies are needed to support this association. In the present study, 1.7% of the patients had meningoencephalitis, whereas Narayanasamy et al. (15), showed a higher incidence rate (9%). The pathognomonic sign of this disease is the presence of organisms in CSF due to invasion and inflammation of leptomeninges. Although many studies have documented GI involvement in scrub typhus, the exact cause remains unclear.
Neurological symptoms, such as altered sensorium, were found to be more prevalent in children, which might be due to dyselectrolytemia, hypoxia, sepsis, and hypoglycemia. Moreover, 43.6% of children had elevation of SGOT fivefold (P < 0.001), whereas there was no case of hepatitis, probably due to mild portal inflammation, without causing significant hepatitis. The main causes of CNS involvement in scrub typhus were direct invasion of organisms across the cerebrospinal fluid (CSF) barrier and small vessel vasculitis changes in the meninges (18).
The serum albumin level was low in patients with severe scrub typhus (OR = 3; CI = 1.427 to 7.110; P < 0.006), which is probably due to increased capillary leakage from blood vessels, leading to hypoalbuminemia (19, 20). This association seems to be an independent risk factor for the severity of disease, as demonstrated by Lee at al. (21), and Kim et al. (20). In our study 69.2% of patients with severe scrub typhus had thrombocytopenia with platelet counts below 1.5 lakh/cumm. However, in the present study, there was no cut-off point for severe thrombocytopenia, unlike other studies. In addition, the results showed that 59% of patients with severe scrub typhus had anemia (Hb < 10 g/dL). Karanth et al. (14), also found that 47.4% of patients had anemia, which is one of the predictors of disease severity (Hb < 12 g/dL). Nevertheless, Kim et al., could not find such an association.
The mortality rate was 1.3% in our population, which is similar to the rate reported by Kim et al. (11), (0.5%). On the other hand, Varghese et al. (5), reported a mortality rate of 14% from India, and Watt et al. , reported a mortality rate of 15% - 30% from Thailand.
Based on the analysis of studies on the severity of scrub typhus (Table 6), some parameters were found to be common, including anemia, thrombocytopenia, and hypoalbuminemia, while other parameters, such as age, leukocytosis, eschar, multi-organ dysfunction syndrome, and serum creatinine could not be compared, most likely due to the insignificant number of cases. It was concluded that low hemoglobin level, thrombocytopenia, transaminitis, low albumin level, and prolongation of prothrombin time were the major predictors of scrub typhus severity in the present study.
| Authors | Journals | Number of Patients | Number of Severe | Number of Non-Severe | Severity Predictors | Odds Ratio | P Value |
|---|---|---|---|---|---|---|---|
| Narayanasamy et al. (15) | Indian Journal of Pediatrics | 230 | 91 | 133 | Breathlessness | Breathlessness (OR = 6.85; 95% CI = 2.69 - 9.87) | 0.009 |
| Altered sensorium | Altered sensorium (OR = 11.48; 95% CI = 3.43 - 10.19) | 0.001 | |||||
| Leukocytosis | Leukocytosis (OR = 3.38; 95% CI = 1.12 - 10.16) | 0.03 | |||||
| Hypoalbuminemia | Hypoalbuminemia (OR = 10.78; 95% CI = 2.66 - 48.76) | 0.001 | |||||
| Hyponatremia | Hyponatremia (OR = 10.08; 95% CI = 2.11 - 23.42) | 0.001 | |||||
| Pamornsrisriwongpan et al. | Clinical Risk-Scoring Algorithm to Forecast Scrub Typhus Severity | 526 | 100 | 357 | Age < 15 years | Age (OR = 4.09) | < 0.001 |
| Pulse rate | Pulse rate (OR = 3.19) | < 0.001 | |||||
| Crepitation | Crepitation (OR = 2.97) | < 0.001 | |||||
| Serum aspartate aminotransferase level > 60 IU/L, | Serum aspartate aminotransferase (OR = 2.89) | < 0.001 | |||||
| Serum albumin < 3.0 g/dL | Serum albumin (OR = 4.69) | < 0.001 | |||||
| Serum creatinine > 1.4 mg/dL | Serum creatinine (OR = 8.19) | < 0.001 | |||||
| Kim et al. (11) | BMC Infectious Diseases | 333 | 89 | 119 | Age > 60 years, | Age > 60 years (OR = 3.13) | 0.002 |
| Absence of eschar, | Absence of eschar (OR = 6.62) | 0.03 | |||||
| WBC >10,000/mm3 | WBC >10,000/mm3 (OR=3.6) | 0.001 | |||||
| Serum albumin level < 3 g/dL | Serum albumin < 3 g/dL (OR = 5.01) | 0.004 | |||||
| Varghese et al. (5) | International Journal of Infectious Diseases | 623 | 212 | 411 | Hypotension requiring inotropes, | Hypotension requiring inotropes (OR = 10.1) | < 0.001 |
| Creatinine > 2 mg/dL | Creatinine > 2 mg/dL (OR = 3.5) | 0.001 | |||||
| CNS dysfunction | CNS dysfunction (OR = 6) | < 0.001 | |||||
| Bilirubin level > 2 mg/dL | Bilirubin > 2 mg/dL (OR = 1.4) | 0.36 | |||||
| Ventilation | Ventilation (OR = 2.2) | 0.14 | |||||
| Karanth et al. (14) | Asian Pacific Journal of Tropical Disease | 220 | 116 | 104 | Hemoglobin < 12 g/dL | Hemoglobin < 12 g/dL (OR = 2) | 0.037 |
| WBC > 10,000 cells/mm3 | WBC > 10,000 cells/mm3 (OR = 3) | 0.001 | |||||
| Platelet count < 150,000 cells/mm3 | Platelet < 150 000 cell/mm3 (OR = 6.2) | < 0.001 | |||||
| Absence of eschar | Absence of eschar (OR = 0.4) | 0.01 |
Comparison of Studies on Scrub Typhus
5.1. Limitations
First, since the rate of mortality was low, it was not possible to determine the risk factors for mortality. Second, it was not possible to determine the serotypes of scrub typhus, and therefore, the association between disease severity and genotypes was not evaluated. Third, considering the retrospective design of this study, it was not possible to identify other co-infections. Finally, diagnoses were clinically established based on serological tests, and they were not confirmed by the gold standard test (PCR assay).
5.2. Conclusions
The predictors of disease severity in the present study were anemia, hypoalbuminemia, thrombocytopenia, prolongation of prothrombin time, elevation of SGOT, and GI symptoms. The presence of one or more of these clinical and biochemical markers should raise the physician’s suspicion about the severity of the disease, warranting immediate and comprehensive treatment in view of the possible complications and highlighting the need for parental counseling about the disease.