1. Background
Although Hospital-acquired Infection (HAI) in Neonatal Intensive Care Units (NICUs) has been known for many years, it is still one of the main causes of morbidity and mortality in these units. The prevalence of HAI in NICUs is estimated to be about 30% that is higher in developing countries (up to 40%), leading to infant mortality. Hospital-acquired infection increases the risk of severe neurodevelopmental disorders, especially in premature infants, the length of hospital stay, and hospital costs (1).
Newborns are susceptible to HAI due to the lack of immune system evolution and the immaturity of innate barriers such as the skin. They are also exposed to other sick infants and a variety of interventions and medications such as broad-spectrum antibiotics and anti-acids. On the other hand, the utilization of different devices and catheters, such as tracheal tubes, Central Venous (CV) lines, chest tubes, and other equipment, is inevitable as part of advanced therapeutic interventions in NICUs to save the lives of patients. However, these procedures can also increase the rate of infection. Each of these invasive interventions can contribute to HAI, depending on the aseptic condition, methods of care, and infection control policies in every health facility (2).
The symptoms of infection in infants are usually nonspecific, and deciding to use broad-spectrum antibiotics is difficult. Therefore, a good knowledge of epidemiology and the incidence of common germs in each ward can help to make the right decisions to reduce and control HAI. The periodic, frequent survey of HAI, based on a reliable data collection system and analysis, will result in evidence-based decision-making and hence, better planning and executing nosocomial infection control programs (3).
Although the prevalence of infections and common microorganisms and factors affecting the incidence of HAI have been widely studied, the results are highly influenced by factors such as patient’s general conditions, staff-to-patient ratio, the number of interventions, etc. Therefore, regular evaluation of HAI is necessary for every NICU.
2. Objectives
We studied the prevalence of HAI, the site of infection, responsible infectious organisms, and relationships with risk factors in a NICU (4).
3. Methods
A descriptive cross-sectional study was conducted for a year in the NICU of Ali Asghar Hospital affiliated to the Iran University of Medical Sciences. This level-III NICU is a referral center with 30 active beds that accommodate infants with surgery, respiratory, endocrine, and metabolic disorders, and prematurity. All infants who were admitted from December 2015 to December 2016 and stayed for at least 48 hours at the NICU were included in the study, until discharge or death.
According to previous studies, risk factors for Healthcare-associated Infections (HAIs), which were considered as variables in this research, included Endotracheal Intubation (ETT), mechanical ventilation, urinary catheter, chest tube, any type of drain in the chest and abdomen, central venous catheter, Peripherally Inserted Central Catheter (PICC), umbilical cord catheter, surgery, staff-to-patient ratio, infant weight at admission, and duration of hospital stay. The criteria used for diagnosing HAI was based on the definition of the Centre for Disease Control and Prevention (CDC) and the National Nosocomial Infections Surveillance (NNIS) system. All cases of HAI were confirmed by a pediatric infectious disease subspecialist who was in charge of the infection control committee of the hospital. Since the hospital was not a maternity center admitting referral patients, HAI was described as any of the following observations:
(1) Urinary Tract Infection (UTI) with positive urine culture
(2) Blood Stream Infection (BSI) with positive blood culture while having a negative blood culture in the first 48 hours of hospitalization. In the absence of positive blood culture, clinical sepsis was also considered as HAI if there were two clinical signs or positive laboratory symptoms or one clinical sign plus one laboratory symptom. Clinical signs included malaise, lethargy, poor feeding, reduced neonatal reflexes, seizure, diarrhea, vomiting, hypo/hyperthermia, unexpected icterus, abdominal distension, tachypnea, respiratory distress, and antibiotic change by the physician. The laboratory criteria included the WBC count of less than 5000 or more than 15,000, the platelet count of less than 100,000, the blood sugar of less than 45 mg/dL, CRP of at least 2+, the CSF analysis consistent with meningitis, and positive CSF culture.
(3) Pneumonia, diagnosed by the instability of respiratory state with new infiltration in the chest X-ray or the necessity of increasing respiratory support under mechanical ventilation.
(4) Surgical site infection suspected based on leakage from the surgical wound or drains and proved based on a positive culture of wound secretions or re-opening of the wound.
Data collection tools consisted of a demographic questionnaire, the monthly report of the hospital infection control committee based on the NNIS, and the daily chart of all risk factors for each newborn hospitalized in the NICU, collected by two colleague researchers. The data used in this study were obtained from the hospital committees and Hospital Information System (HIS) that were annually collected and used to plan internal hospital policies. Hence, there was no need for the approval of the Ethics Committee.
3.1. Statistical Analysis
In this study, STATA software was used for data analysis. The frequency and percentage were used for qualitative data analysis and the mean and confidence intervals for quantitative data analysis. Finally, the mean values of the two groups (infected vs. non-infected) were compared using the independent t-test and chi-square frequency test. The relationships between the risk factors and the incidence of HAI were investigated based on logistic regression reported by the odds ratios with confidence intervals.
4. Results
In this research, 654 newborns (9296 patients/day) were considered as prototypes in one year. The numbers of neonates without HAI and infants suffering HAI were 566 (86.5%) and 88 (13.5%), respectively. Among patients with nosocomial infections (n = 88), 71 (80.7%) cases had bloodstream infections, including 64 (72.7%) cases of clinical sepsis and seven (8%) cases of positive blood cultures. Other cases exhibited pneumonia (9%), surgical site infection (8%), and urinary tract infection (2.3%) (Table 1). The average time to the occurrence of HAI was 13.5 days after admission.
| Localization | Infection Frequency | Infectious Agents | N | |
|---|---|---|---|---|
| N | % | |||
| BSI-LCBI | 7 | 8 | Candida albicans | 3 |
| Streptococcus | 1 | |||
| Escherichia coli | 1 | |||
| Acinetobacter baumannii | 1 | |||
| Staphylococcus epidermidis | 1 | |||
| CLABSI | 64 | 72.7 | ||
| Pneumonia | 8 | 9 | Acinetobacter baumannii | 7 |
| Klebsiella | 1 | |||
| Surgical Site Infection | 7 | 8 | Acinetobacter baumannii | 6 |
| Escherichia coli | 1 | |||
| Urinary Tract Infection | 2 | 2.3 | Enterococcus | 1 |
| Escherichia coli | 1 | |||
| Total | 88 | 100.0 | ||
Abbreviations: BSI-LCBI, blood stream infection-laboratory-confirmed bloodstream infection; CLABSI, central line blood stream infection
The comparison of qualitative variables between the two groups revealed that the frequency of female sex was 38.6% (n = 34) in the infected group and 39.8% (n = 225) in the non-infected group. On the other hand, the frequency of male gender in the groups with and without infection was 61.4% (n = 54) and 60.2% (n = 340), respectively. These differences between the two groups were not statistically significant (P = 0.907). The comparison of the frequency of surgery history between the groups with and without infection showed that the frequency of surgical history was 34.1% (n = 30) in the infected group and 6.7% (n = 39) in the non-infected group, showing a statistically significant difference (P = 0.0001). The frequency of cases in different weight ranges including below 1,000 g, 1,001 to 1,500 g, 1,501 to 2,500, and equal to or above 2,501 g in the infected group was 2.6%, 6.9%, 21.4%, and 69.1%, respectively; The values were significantly different from those in the non-infected group (P = 0.0001) (Table 2).
| Variables | Total (%) | Infection, No. (%) | P Value | |
|---|---|---|---|---|
| Yes (N = 88) | No (N = 566) | |||
| Sex | 0.907 | |||
| Female | 259 (39.6) | 34 (38.6) | 225 (39.8) | |
| Male | 395 (60.4) | 54 (61.4) | 340 (60.2) | |
| Surgery | 0.0001 | |||
| Yes | 73 (11.2) | 30 (34.1) | 39 (6.7) | |
| No | 581 (88.8) | 58 (61.4) | 527 (93.1) | |
| Weight, g | 0.0001 | |||
| < 1000 | 17 (2.6) | 3 (3.5) | 14 (2.6) | |
| 1001 - 1500 | 44 (6.7) | 7 (8.1) | 37 (6.9) | |
| 1501 - 2500 | 135 (20.6) | 20 (23.3) | 115 (21.4) | |
| > 2500 | 427 (65.3) | 56 (65.1) | 371 (69.1) | |
| Nationality (Iranian) | 0.003 | |||
| Yes | 601 (91.9) | 88 (100) | 513 (90.6) | |
| No | 53 (8.1) | 0 (0) | 53 (9.4) | |
| Outcome | 0.567 | |||
| Discharge | 616 (94.2) | 82 (93.2) | 534 (94.7) | |
| Death | 36 (5.5) | 6 (6.8) | 30 (5.3) | |
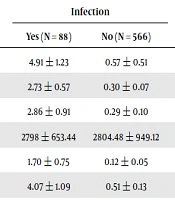
The quantitative variables were compared between the groups with and without infection based on mean values. According to Table 3, the duration of endotracheal tube use was 4.91 ± 1.23 days in the infected group and 0.57 ± 0.51 days in the non-infected group. The results of urinary catheter and chest tube in the infected group were significantly higher than those in the non-infected group (2.73 ± 0.57 vs. 0.30 ± 0.07 for urinary catheter and 2.86 ± 0.91 vs. 0.29 ± 0.10 for chest tube). On the other hand, the numbers of days having a CV line, cut-down catheter, and PICC catheter were 1.70 ± 0.75, 4.07 ± 1.09, and 0.61 ± 0.15, respectively, in the infected group, which were significantly higher than those in control (non-infected) group (0.12 ± 0.05, 0.51 ± 0.13, and 6.69 ± 1.57, respectively) (P = 0.0001) (Table 3). On the other hand, the mean weight and the number of admission days were 273.64 ± 2798 and 35.93 ± 23.95, respectively, in the infected group. There was a significant difference between the infected and non-infected groups in terms of the number of hospitalization days (P = 0.0001) (Table 3).
| Variables | Infection | P Value | 95% CI | ||
|---|---|---|---|---|---|
| Yes (N = 88) | No (N = 566) | Lower | Upper | ||
| ETT (days) | 4.91 ± 1.23 | 0.57 ± 0.51 | 0.0001 | -5.44 | -3.22 |
| Urinary catheter (days) | 2.73 ± 0.57 | 0.30 ± 0.07 | 0.0001 | -3.00 | -1.84 |
| Chest tube (days) | 2.86 ± 0.91 | 0.29 ± 0.10 | 0.0001 | -3.44 | -1.71 |
| Weight (g) | 2798 ± 653.44 | 2804.48 ± 949.12 | 0.951 | -199.47 | 212.38 |
| CV line (days) | 1.70 ± 0.75 | 0.12 ± 0.05 | 0.0001 | -4.63 | -2.47 |
| Cut-down (days) | 4.07 ± 1.09 | 0.51 ± 0.13 | 0.0001 | -7.52 | -4.64 |
| PICC (days) | 0.61 ± 0.15 | 6.69 ± 1.57 | 0.002 | -0.77 | -0.22 |
| Length of stay (days) | 35.73 ± 23.95 | 11.02 ± 9.55 | 0.0001 | -13.09 | -8.77 |
Abbreviations: CV line, central venous line; ETT, endotracheal intubation; PICC, peripherally inserted central catheter
Logistic regression was used to investigate the risk factors associated with the incidence of hospital infections. Table 4 shows that infants with a history of surgery were 3.65 times more likely to have nosocomial infections than children who have no history of surgery (OR: 3.65, 95% CI: 1.71 - 7.78). Moreover, for each day increase in hospitalization, the risk of hospital infections increased by 10% (OR: 1.10, 95% CI: 1.08 - 1.12). On the other hand, having a CV line for each additional day increased the chance of infection in infants by 12% (OR: 1.12, 95% CI: 1.01 - 1.16).
| Variables | β | SE | Adjusted OR | P Value | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Surgery (yes/no) | 1.296 | 0.385 | 3.65 | 0.049 | 1.71 | 7.78 |
| Urinary catheter | 0.098 | 0.050 | 1.10 | 0.001 | 1.00 | 1.21 |
| Length of stay (days) | 0.100 | 0.010 | 1.10 | 0.0001 | 1.08 | 1.12 |
| ETT | 0.091 | 0.025 | 1.09 | 0.0001 | 1.04 | 1.14 |
| Chest tube (days) | 0.070 | 0.031 | 1.07 | 0.024 | 1.00 | 1.13 |
| CV line | 0.081 | 0.036 | 1.12 | 0.025 | 1.01 | 1.16 |
Abbreviations: CV line, central venous line; ETT, endotracheal intubation
5. Discussion
Hospital infection is known as an important factor in increasing morbidity, mortality, and the number of patients in NICUs. In addition to the underlying illness of neonate, several factors increase the chance of hospital infection, including prematurity, low birth weight, length of hospital stay, facilities, intervention types, and implementation method (5). In recent years, some researchers have focused on hospital infection prevalence and presented a wide range of influential factors. These differences may be due to the differences in applied statistical procedures, definitions of hospital infection, structural characteristics of hospital sections, the type of patients admitted to each center, and changes in the patterns of microbial resistance over the years. Therefore, in this research, we tried to provide the most statistical and up-to-date information.
The prevalence of hospital infection in our study was 13.5%, while it was estimated at 3.9% in a study by Brack et al. (6). Darvishpour et al. (7) reported a prevalence of 16.9% in a one-year study in 2010. In a study from Egypt in 2013 (8), a prevalence of 21.4% was reported, and pneumonia was the most common type of HAI (11.3%), followed by positive blood culture (8.8%) and urinary tract infection (3.1%). Although in this study, the research method and duration of hospital stay in infected and non-infected patients were very similar to those of our research, the mortality rate was not significantly different between infected patients (29.6%) and non-infected patients (24.5%). In a study performed in 2008 by Tavora et al. (9), using a method very similar to our research method, this rate was reported to be 34%. In this study, the number of infected cases based on the diagnosis approach was reported as 47.2% (clinical findings), 20.9% (positive blood culture), 8.6% (pneumonia), and 2.4% (surgical wound infections). In this study, the ratio of nurse-to-patient was similar to that of our study.
A study was performed in Tehran (10) to investigate the number of nosocomial infections in ICUs among children and infants in a pediatric medical center. The infection rate was estimated at 12.2%. The mean duration of hospitalization was 13.1 days in the infected group. In a study by Aziz et al. (11) in 2005, based on information from 17 level-III NICUs, the mortality rate was significantly higher in hospitalized infected neonates (8.5%) than in infants without infection (1.3%). However, this was not the case for infants weighing less than 1,500 grams (8.7% in infected infants and 8.6% in non-infected infants). In this research, the most important risk factors of independent hospital-related infections were low gestational age, mechanical ventilation, and intravenous lipid administration in neonates weighting less than 1,500grams and gestational age, use of central venous pathways and peripheral venous pathways, intravenous injection of lipids and amino acids, and mechanical ventilation in newborns above 1,500 grams. In Yue et al. study in China (12) conducted for five years in ICUs, the prevalent microorganisms in the NICU were Acinetobacter bumanni, and Escherichia coli and the incidence of Acinetobacter was higher in patients who had surgery in the special unit. In an Egyptian study (13) in 2017, the common microorganism was Klebsiella, followed by Escherichia coli. The density of hospital infection (Incidence Density) (number of NI / number of patient/day) x 1000 was in China 1000 / 12.5, in Australia 10,000 / 5, in Colombia 1000 / 6.2, in Italy 1,000 / 6.93, in America 1,000 / 6,9 - 8,9, in Egypt 1000 / 13.8 in Turkey was 1000/18.1, and in our research was 9.46 / 1000 (14, 15). The average incidence rate of hospital infection was 13.5 days after admission, and the average total accommodation was 14.3 days. In a Korean study (16) in 2006, the average incidence rate of hospital infection was 16.5 days after admission, and the average length of stay was 22 days. In the Perak study (17) on the risk factors of hospital infection, significant relationships were observed with hospital type, use of suction (P = 0.05), and surgery (P = 0.04). In a study by Fujimura et al. (18) in 2006 on 1,050 infants, strong predictors for the risk of hospital infection were the length of staying in the unit, duration of mechanical ventilation, and duration of CVCs. In a study in India (19), the most important risk factors for the incidence of hospital infection were low gestational age, male gender, the use of intravenous routes, and the duration of mechanical ventilation. The mortality rate of infected neonates was 29% in the above-mentioned research and 6.8% in our study, while the mortality rate due to hospital infections is 20% to 80% depending on risk factors.
Overall, the prevalence of hospital infection varies widely in different countries and even across a country. It seems that our situation is worse than the status of developed countries but better than in some developing countries. Culture-negative clinical sepsis and lack of follow-up data were the most important limitations of this study.
5.1. Conclusions
Invasive measures and procedures in NICUs have a significant relationship with the rate of hospital infection. Therefore, limiting these efforts, trying to do the right procedures, and complying with the standard procedures can help better control hospital infection.
5.2. Research Constraints
According to research, one of the important risk factors for hospital infection in NICUs is complete intravenous nutrition, which was not included in this study because the approach in most cases was the therapeutic intervention. In addition, since the study setting was a referral hospital, data related to gestational age, delivery, Apgar Score, and maternal problems during pregnancy were not collected, which made it impossible to compare the results with other similar studies. In addition, in the Iran National Monitoring System, only four groups, including urinary tract infections, surgical wounds, respiratory and hematopoietic infections were selected from the total number of NNIS codes and reported. Cellulitis, abscess, omphalitis, conjunctivitis, and necrotizing enterocolitis were not considered as hospital infections, and therefore our obtained infection rates may differ from those of other studies. Moreover, in addition to risk factors assessed in this study, other risk factors cloud affect the incidence of hospital infection, such as the type of nutrition and routine care, which were not investigated in this study. It is suggested that in future studies, hospital infection rates be considered with all NNIS codes to obtain more accurate statistical data for NICU hospital infection.