1. Background
As one of the most common systemic vasculitis types, one can mention Henoch-Schönlein purpura (HSP) (1). Its symptoms present with non-thrombocytopenic palpable purpura, fever, abdominal pain, arthralgia, arthritis and nephritis. In addition, Myalgia, subcutaneous edema, pulmonary hemorrhage, pancreatitis, orchitis, testicular torsion and CNS vasculitis may occur. Moreover, Eyes and adrenals might be involved (2). Immunoglobulin A (IgA) has an important role in its immune pathogenesis, hence HSP is known as IgA vasculitis (3).
HSP patients were studied in various countries; considering epidemiology, clinical features, lab data and treatment (4-8). Lee et al. (9) (2004 - 2015) studied HSP patients in Korea and reported GI complications, joint and renal involvement in 75.0%, 69.8% and 26.9% of the patients, respectively. Scrotal swelling was reported in 4.7% of the patients. There were two studies in Spain that included different cities in two different time periods. Calvino et al. (10) (1980 - 1999) reported palpable purpura in all of the patients and Joint, GI and renal involvement were reported in 78.2%, 73.1% and 42.0% of the studied patients, respectively. While Calvo-Rio et al. (11) (1975 - 2012) reported cutaneous involvement in all of the patients; GI, joint and renal involvement, leukocytosis, elevated serum IgA level and anemia in 63.1%, 64.5%, 41.2%, 36.7%, 31.7% and 8.9% of the patients, respectively. In Italy, Trapani et al. (12) (1998 - 2002) reported palpable purpura in all of the studied patients. Joint, GI and renal involvement, leukocytosis, anemia, increased ESR and IgA levels were reported in 74%, 69%, 54%, 21%, 14%, 57% and 37% of the patients, respectively. There was a study in Iran, Tehran, about HSP patients with nephritis in a twenty-year period that reported palpable purpura in 100% of the patients; joint, GI and renal involvement in 88.6% 73.3% and 39.0% of the patients, respectively (13).
HSP is a benign condition and the treatment is symptomatic; however, there are studies that reported the effectiveness of corticosteroids in the reduction of life-threatening complications of HSP such as renal involvement (14). Prednisolone is the common steroid in HSP treatment, used as 1 mg/kg/day for 1 - 2 weeks (15). Still, the effectiveness of corticosteroids in HSP is controversial (16).
Since HSP involves multiple organs and some of its life threatening complications, such as renal involvement, occur during time; long term follow up of the patients is suggested (17). Epidemiology and clinical presentations of HSP are affected by genetics, ethnicity and environment (3, 14). As we found few studies on HSP in southwest of Iran, clinical and demographic features; as well as lab data are reported here. Presented data are compared with the related studies.
2. Objectives
There are few studies that have investigated HSP clinical presentations in southwest of Iran (13). Therefore, this retrospective study was performed to assess demographic and lab data, clinical presentations and treatment outcome of the patients with HSP who were admitted to Namazi hospital (southwest of Iran, Shiraz).
3. Methods
In this retrospective descriptive study, the recorded data of all patients who were hospitalized in Namazi Hospital (the tertiary center in southwest of Iran, Shiraz) with the diagnosis of HSP were evaluated (2006 - 2018).
The Ethics Committee of Shiraz University of Medical Sciences approved this study (Approval ID: IR.SUMS.MED.REC.1397.118).
HSP criteria were evaluated based on European League Against Rheumatism (EULAR) and Pediatric Rheumatology European Society (PRES) (18); and were further validated with Pediatric Rheumatology International Trials Organization (PRINTO) (19).HSP criteria consisted of palpable purpura presented with at least one of the following signs: renal involvement, acute abdominal pain, and acute arthritis, biopsy showing leukocytoclastic vasculitis with predominant IgA deposit or proliferative glomerulonephritis with predominant IgA deposit (19). Patients < 18-year-old, who fulfilled HPS criteria and were admitted to the hospital during 2006 - 2018 were included in this study. Patients with mild HSP who were not admitted; those with other types of vasculitis, drug hypersensitivity or coagulopathy were excluded from the study. In total, 195 patients were observed in this study. Individual medical file records for all patients with HSP diagnosis, who were admitted from 2006 - 2018, were reviewed and retrieved using a standardized study-specific data collection form. Recorded hospitalization data were observed; and outpatient follow up visit data were recorded after 2 weeks of patient discharge.
History, demographic characteristics such as sex, age; the season of admission, hospitalization duration and clinical presentations in physical examination such as: palpable purpura, GI disorders (pain, bleeding, nausea/vomiting), arthralgia, arthritis, myalgia, renal involvement, scrotal edema and other HSP presentations were investigated.
Besides, laboratory findings such as: BUN and creatinine level, platelet, full blood cell count (WBC, leukocyte, neutrophil count, RBC), stool occult blood, CRP level, blood pressure and urine analysis were considered. Proteinuria was noted as 1+ (30 mg/dL), 2+ (100 mg/dL), 3+ (300 mg/dL) and 4+ (100 mg/dL). Hematuria or red blood cell casts: > 5 red blood cells/high power fields were noted. Renal involvement was defined as any sign of hematuria and/or proteinuria, high blood pressure or age related Cr level.
3.1. Statistical Analysis
Statistical analyses were conducted by SPSS 15, to estimate the mean age of the patients, duration of hospitalization, laboratory data and the prevalence of HSP in different seasons. Then, chi-square test was applied in order to compare the relations between age and anemia as well as age and neutrophilia. ANOVA test was performed to check the difference between seasons of HSP presentation and duration of hospitalization. The difference between HSP presentations and duration of hospitalization was checked using ANOVA test, as well. P value < 0.05 was considered as significant.
4. Results
From 195 followed patients, 118 (60.5%) patients were male and 77 (39.5%) were female (Male/female = 1.53). The age of the patients ranged from a minimum of 7 months to maximum of 17 years (mean = 6.7 ± 3.21 years). The duration of hospitalization was 1 - 17 days (mean = 4.55 ± 2.83 days). In seasonal evaluation, 30 (15.4%) patients presented with HSP in spring, 30 (15.4%) in summer, 86 (44.1%) in autumn and 49 (25.1%) in winter. Specific demographic data are shown in Table 1. In this study, when we noted the duration of hospitalization in each season, no statistically significant relationship was observed (P value = 0.085). About 70 (36%) patients showed common cold symptoms about two weeks before showing HSP signs and symptoms. The admission rate of HSP patients was calculated as presented in Table 2.
| Criteria | Value |
|---|---|
| Sex (number of patients (%)) | |
| Male | 118 (60.5) |
| Female | 77 (39.5) |
| Male/Female ratio | 1.53 |
| Age (year) | |
| Mean ± SD | 6.7±3.21 |
| Range | 0.58-17 |
| Hospitalization (days) | |
| Mean ± SD | 4.55±2.83 |
| Seasonal distribution (number of patients (%)) | |
| Spring | 30 (15.4) |
| Summer | 30 (15.4) |
| Autumn | 86 (44.1) |
| Winter | 49 (25.1) |
| Relapse | 8 (4.1) |
| Prednisolone treatment | 84 (43.1) |
| Oral prednisolone | 67 (34.3) |
| IV Methyl prednisolone | 17 (8.7) |
Demographic data of patients With Henoch-Schönlein Purpura
| Year | Number of Admitted Pediatric Patients | Number of Admitted HSP Patients | HSP Admission Rate |
|---|---|---|---|
| 2006 | 6226 | 13 | 0.21 |
| 2007 | 6160 | 14 | 0.23 |
| 2008 | 6135 | 12 | 0.19 |
| 2009 | 7169 | 13 | 0.18 |
| 2010 | 7377 | 21 | 0.28 |
| 2011 | 7423 | 23 | 0.31 |
| 2012 | 7758 | 19 | 0.24 |
| 2013 | 7129 | 26 | 0.36 |
| 2014 | 7246 | 12 | 0.16 |
| 2015 | 7865 | 7 | 0.09 |
| 2016 | 9305 | 16 | 0.17 |
| 2017 | 9321 | 9 | 0.10 |
| 2018 | 10598 | 10 | 0.09 |
The Admission Rate of HSP Patients During 2006 - 2018
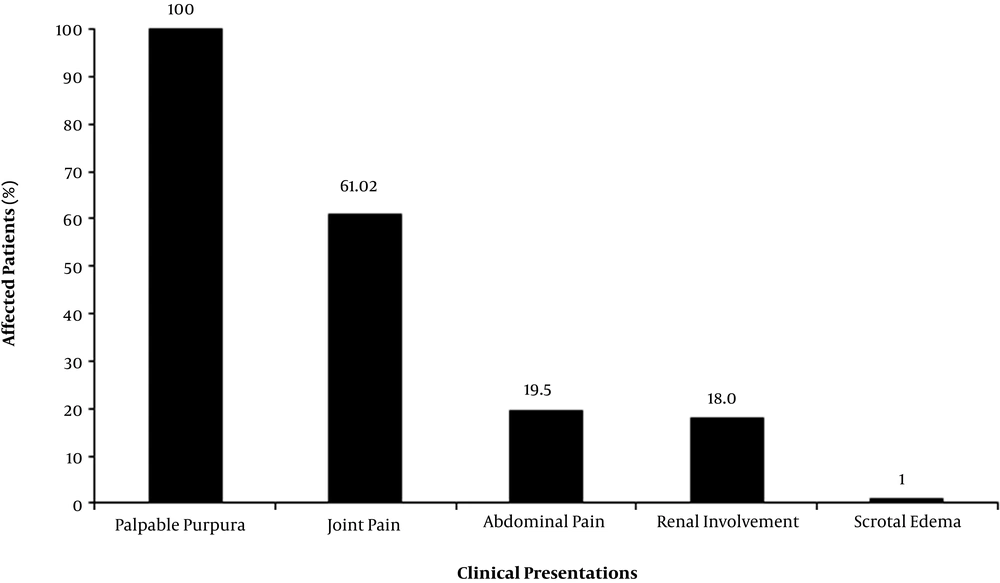
Figure 1 presents the clinical manifestations in percentage. Palpable purpura and joint pain were observed in 195 and 119 patients. Abdominal pain and nausea/vomiting were noted in 38 and 31 patients. The observed abdominal pain was recorded without tenderness. Intussusception was suspected in the primary examination of one patient; however, it was ruled out in further observations. GI bleeding was observed in three patients as hematemesis; and in 7 patients as positive results in stool occult blood (OB) test (total of 5.13). Two patients were observed with high blood pressure (age adjusted data). Renal involvement was observed in 35 patients (considering hematuria, proteinuria). Scrotal edema was noted in two patients; while other clinical signs, such as pancreatitis, pulmonary hemorrhage, myalgia, subcutaneous edema, orchitis, testicular torsion and CNS vasculitis, were not recorded in the studied patients.
Based on the laboratory findings, 106 patients were noted with leukocytosis (mean WBS number = 11.38 * 103/mm3) and 156 patients had neutrophilia (mean neutrophil percentage = 60.92%). Also, 93 patients had anemia (age adjusted data) in their course of disease, and thrombocytosis (platelet count ≥ 500 * 103/mm3) was recorded in 28 patients (mean platelet count = 409 * 103/mm3). Chi-square results revealed no significant correlation between age and leukocytosis (P value = 0.094), as well as with neutrophilia (P value = 0.06); however, anemia was observed to be correlated with age (P value = 0.006). BUN and creatinine (Cr) analyses were used to assess the renal function in the patients. Mean BUN level was 12.30 mg/dL and only 8 patients had BUN > 20 mg/dL. All patients were noted with normal creatinine level with (mean = 0.56 mg/dL). CRP ≥ +1 was noted in 18 patients. Table 3 presents the clinical manifestations and lab data all together.
| Variable | Number of Patients (%) |
|---|---|
| Clinical features | |
| Abdominal pain | 38 (19.49) |
| Joint pain | 119 (61.02) |
| GI bleeding | 10 (5.13) |
| High blood pressure | 2 (1.02) |
| Nausea/vomiting | 31 (15.90) |
| Palpable purpura | 195 (100.00) |
| Renal involvementa | 35 (17.95) |
| Scrotal edema | 2 (1.02) |
| Lab data | |
| Leukocytosis | 106 (54.36) |
| Neutrophilia | 156 (80.00) |
| Anemia | 93 (47.69) |
| Thrombocytosis | 28 (14.36) |
| Positive OBT | 7 (3.59) |
| BUN > 20 mg/dL | 8 (4.10) |
| Cr > 1.5 mg/dL | - |
| Hematuria | 23 (11.79) |
| Proteinuria | 12 (6.15) |
| CRP | 18 (9.23) |
Clinical Presentations and Lab Data
The correlation between HSP presentations and duration of hospitalization was evaluated. It was observed that the difference of hospitalization duration with WBC count (P value = 0.031) and OB test (P value < 0.001) was significant; However, the difference with other clinical symptoms (GI, joint and renal involvement; anemia, platelet count, Cr level, BUN level) was not significant.
Oral prednisolone was prescribed for patients with GI symptoms (abdominal pain, GI bleeding, nausea, vomiting), arthritis, renal involvement and patients with progressive cutaneous presentations. In case that the patient could not tolerate oral prednisolone (presented with nausea, vomiting, GI bleeding) intravenous methyl prednisolone was applied. Of 195 patients, 84 patients received prednisolone. Seventeen patients needed intravenous methyl prednisolone pulse therapy for 3 days; while, the rest of the patients received oral prednisolone (1 mg/kg) for 7 - 10 days.
Eight patients were admitted due to recurrence of HSP symptoms, that were not complicated, and they were treated by oral prednisolone. Renal function and other clinical and lab presentations were recorded as normal at hospital release or during outpatient follow up.
5. Discussion
Total of 195 patients were hospitalized due to HSP in the study period (2006 - 2018). Males outnumbered females and the ratio of males to females was 1.53, which is compatible with previous findings that reported higher incidence in male patients (9, 20). The mean age of patients in our study was 6.7 ± 3.21 years. Lee YH et al. reported the age of 6.39 years in Korean patients (9) and Nickavar et al. reported the mean age of 73.0 ± 33.4 months in Iranian patients in Tehran Province (13).
The duration of hospitalization was 1 - 17 days. In seasonal evaluation, the highest frequency of HSP cases was recorded in autumn. In previous reports, autumn and winter were mentioned to be the seasons with the highest incidence of HSP (21, 22). Moreover, a study in Spain showed that autumn is the season with the highest frequency of HSP records (10)) that is in line with the present study. However, studies in Turkey indicated that most of the HSP cases are observed in winter (7). The reported correlation factors with HSP in literature include: infections, vaccination, medicines, tumors, insect bite and food (17). The higher HSP prevalence in autumn can be due to higher load of infections in our region in this season (14).
Evaluation of clinical features in our study revealed that the major detected sign was palpable purpura, as necessary diagnostic criteria, consistent with other studies (7-9, 18-20, 23). In the study by Nickavar et al.; palpable purpura (100%), joint involvements (88.6%) and GI involvements (73.3%) were recorded as clinical features with the highest frequencies (13). In this study, joint pain (61.02%) and abdominal pain (19.49%) were the prominent clinical presentations, as well. Moreover, in the present work, renal involvement was observed in 17.95% of patients. This number is comparable with a previous study that indicated 20% - 54% of renal involvement (24).
Leukocytosis was present in 54.36% of our patients. The rate of leukocytosis presentation here, was higher than the reported data by Calvo-Rio, et al. study in Spain, who observed 36.7% leukocytosis in HSP children (11). Besides, 75% of patients had neutrophilia. Anemia was the other factor that was evaluated in the laboratory study and was found to be correlated with age. Anemia was detected in 47.69% of the cases, which is higher than the prevalence in some other studies (8.9%) (11). Presentation of anemia (age adjusted) was correlated with age, where its P value was particularly significant (P value = 0.006), which again warrants further research. The observed differences with other studies in clinical presentations might be due to genetic and ethnicity differences.
Supportive and symptomatic treatments are recommended for HSP as a self-limited disease. However, in case that hematuria and proteinuria are observed, follow up is needed for at least 6 months, till urine analyses are normal. If nephritic or nephrotic syndrome is developed, pediatric nephrologist should be consulted (2). The application of corticosteroids and immune suppressants in HSP treatment is not exactly clarified. However, corticosteroids were reported to be effective in abdominal pain (GI vasculitis), persistent purpura, decreased levels of coagulation factor XIII and arthritis; that are associated with renal complications (14, 25). Although, Huber’s study showed no statistically significant effect of prednisolone in the risk of severe complications or duration of symptoms improvement (26); Kaku et al. and Calvino et al. concluded that patients with risk factors for renal involvement should be treated with corticosteroids to prevent renal impairment (10, 15). In our study, no complications were reported during follow up period in patients who were treated with corticosteroid.
The limitations of this study included small sample size, short term follow of the patients, retrospective nature of the study and single centre survey. Also, the hospitalized patients’ data, that include the patients with moderate to severe HSP, were included in the present work. While, in most patients; mild cases do not need to be hospitalized. Long-term organ involvement and the role of viral infection in HSP development may be considered in further investigations.
5.1. Conclusion
Based on the presented data, it was observed that males were more prone to HSP, than females; and autumn was the season with the highest prevalence of HSP. Joints and GI were the most involved organs in HSP and the disease complications were ruled out following the treatment. However, long terms follow up is suggested for patients with renal involvement. This study delivers information for HSP diagnosis and complications of the hospitalized patients; that is a motivation for further studies. Besides, the course of disease in other patients, who present with similar clinical features, might be speculated from the results of this study.