1. Background
Acinetobacter baumannii (A. baumannii) and Pseudomonas aeruginosa (P. aeruginosa) are two opportunistic pathogens that have become the sources of grave concern because of their escalating resistance toward diverse antibiotics, leading to the development of multidrug resistance (MDR) or extensive drug resistance (XDR) (1-6). To stress upon the growing global resistance to antimicrobial agents, the World Health Organization (WHO) drew up a list in a bid to guide and promote research and development of new antibiotics in the year 2017 (7) and placed these two microorganisms resistant to carbapenems, the last resort of therapeutic agents, as critical with first priority among others. The search for new antibiotics is time-consuming and requires tremendous economic and labor investment. Moreover, if antibiotics are administered at high doses, toxicity is another constraint (8). Thus, researchers are in pursuit of alternative strategies, which may not generate toxic and adverse effects. Nanoparticles (NPs) may be an approach for effective drug development and delivery against infections caused by MDR bacteria (9-11), owing to their unique physical and chemical properties (8).
The key advantages of NPs in biomedical applications are that they can be easily transported tagged to the diagnostic and therapeutic drugs or biomolecules for targeted cells, due to their unique chemical and physical properties. These distinctive properties makes them suitable for diagnosis, imaging, and therapeutic applications in infectious diseases (12). Indeed, NPs possess antimicrobial activity that can overcome common resistant mechanisms, particularly, when they are combined with antibiotics and act synergistically (13-15). This combination provides complex antimicrobial mechanisms to overcome antibiotic resistance (8). Due to the physical and chemical characteristics of NPs, including surface charges, small sizes, and high surface areas, they can reduce the toxicity and increase the time of drug release and pharmacokinetics for targeted cells (16, 17). Chemical covalently alteration of NPs with sulfide, amine, or carboxyl groups makes them desirable to attach to biomolecules (18). Nanoparticles by the production of reactive oxygen species can damage bacterial cell wall/membrane via blocking the respiratory chain reactions. They also inhibit DNA synthesis and activity of bacterial enzymes by interacting with the phosphorous part of DNA and sulfur of enzymes (16, 19, 20). Given that nanotechnology has unlocked new promises, NPs such as silver (Ag), gold (Au), chitosan, and copper (Cu) have been attempted either alone or in combination against MDR bacteria to overcome antibacterial resistance (3, 21, 22).
Silver has had medical usage since ancient times. Silver NPs possess the potential of antibacterial features targeting diverse bacterial structures, disrupting the cell permeability and respiration by attacking the cell membrane or causing DNA damage by efficiently reacting with phosphorus and damage to other intracellular materials by inducing free radical formation (23, 24). Although AgNps show excellent antimicrobial activity, silver-resistant bacteria are reported to have developed resistance by genetic alterations (25, 26). Chitosan, a deacetylated derivative of chitin, is a non-toxic bacteriostatic agent effective against a broad spectrum of microorganisms (15, 22, 27-31) and considered superior against Gram-negative bacteria in comparison with Gram-positive organisms (27).
2. Objectives
We aimed to assess the effect of covalently bound NPs with either gentamicin or ciprofloxacin on MDR strains of A. baumannii and P. aeruginosa using the microdilution broth and agar dilution methods. The impact of these NPs with and without antibiotics was analyzed by the relative expression of efflux pump genes adeB and mexB for A. baumannii and P. aeruginosa, respectively, using real-time PCR.
3. Methods
3.1. Preparation of Silver Nanoparticles and Their Conjugation with Ciprofloxacin and Gentamicin
Silver nanoparticles (AgNPs) were prepared by a slight modification in the silver colloid preparation method, as described previously by using AgNO3 (Merck, Germany, 101510) (32). The characterization of NPs was performed by transmission electron microscopy (TEM) and electrokinetic measurements. The TEM images were made at an accelerating voltage of 200 kV (TEM, Leo 906, Zeiss, 100KV, Germany). The zeta potential of NPs was measured with a high-throughput Dynamic Light Scattering (DLS) instrument (Malvern, Zetasizer Nanosize ZN3500, England). Silver nanoparticles were combined with ciprofloxacin and gentamicin to produce silver nanoparticles with ciprofloxacin (ANC) and silver nanoparticles with gentamicin (ANG), respectively, as described previously (33).
3.2. Preparation of N,O-Carboxymethyl Chitosan Nanoparticles and Their Conjugation with Ciprofloxacin and Gentamicin
N,O-carboxymethyl chitosan (NOCC) was synthesized by a minor alteration in the amine and oxygen groups of chitosan (Sigma-Aldrich, Germany, 448877), as described previously (34). Briefly, N,O-CMC NPs were prepared by the addition of 1 ml of 0.25% Penta-sodium Tripolyphosphate (TPP) (Merck, Germany, 106999) as the ionic cross-linking aqueous solution to 10 mL of 0.1% N,O-CMC solution under constant stirring at room temperature for 30 min. The prepared NPs were obtained by centrifugation for 45 min at 12,000 g and then lyophilized for further use (35). The characterization of NPs was performed by TEM and Electrokinetic measurements. The TEM images were made at an accelerating voltage of 200 kV. The zeta potential of NPs was measured with the high-throughput DLS instrument. Next, chitosan NPs were combined with ciprofloxacin and gentamicin to produce CNC (chitosan nanoparticles with ciprofloxacin) and CNG (chitosan nanoparticles with gentamicin), respectively (33).
3.3. Bacterial Strains and Antibacterial Assay
The antibiotic-resistant and efflux pump-harboring A. baumannii strain (ATCC 19606) was furnished by Dr. Vajihe Sheikhalizadeh, Department of Bacteriology and Virology, Tabriz University of Medical Sciences, Iran. The MDR A. baumannii (A1, A2, and A3) and P. aeruginosa (P1 and P2) strains along with two non-MDR P. aeruginosa (P3 and P4) strains were from our earlier clinical study (36). Pseudomonas aeruginosa strain PAO1, kindly provided by Dr. Hamid Goli, Department of Microbiology and Immunology, Mazandaran University of Medical Sciences, Iran, was used as a positive strain for the presence of efflux pump. These strains were stocked at -80°C in Tryptic soy broth with glycerol for further use. The antibacterial activity of synthesized NPs against bacteria was evaluated by three different antimicrobial tests, including disc agar diffusion method, drop agar diffusion method, and microdilution broth (37). The MICs of ciprofloxacin and gentamicin were calculated before and after exposure to NPs using the microdilution broth method. Initially, A. baumannii and P. aeruginosa cultures (1.5 × 105 CFU/mL) grown in 100 µL of Mueller-Hinton broth were exposed to chitosan, CNC, CNG, AgNPs, ANC, ANG, gentamicin, and ciprofloxacin at their sub-inhibitory concentrations (0.5xMIC) and incubated for 24 h at 37°C. The MIC was defined as the lowest concentration of NPs that prevented the visible growth of A. baumannii and P. aeruginosa, as described previously (38).
3.4. Expression of Efflux Pump Genes by Real-Time PCR (Quantitative RT-PCR)
In this study, changes in the expression of efflux pump gene adeB in A. baumannii and MexB in P. aeruginosa before and after exposure to sub-MIC of each synthesized materials were measured using real-time PCR. Briefly, the bacteria were cultured in Mueller-Hinton broth at 37°C for 24 h. After incubation, RNA was extracted using an RNA extraction kit (Sinaclon Co., Tehran, Iran), followed by purification by RNase-free DNase I and determination of RNA concentration by a NanoDrop spectrophotometer (Epoch). Finally, RNA was stored at -80°C. The reverse transcription method was used to synthesis cDNA by using the Takara cDNA synthesis kit and finally stored at 4°C. Quantitative real-time PCR was carried out by a Real-Time PCR system (step one version 2.3) using SYBR premix (Takara) and specific primers for adeB and MexB. Besides, 16srRNA was used as the housekeeping gene to normalize the expression of the target genes (39-41). The primers are shown in Table 1. The PCR condition for the amplification of adeB was as follows: 95°C for 2 min and 40 cycles of 10 s at 95°C and 1 min at 60°C. The amplification of MexB was carried out in a three-step method comprising 95°C for 5 min, followed by 40 cycles, each for 20 s at 95°C, 15 s at 64°C, and 15 s at 72°C (42, 43). The PCR condition for the amplification of 16srRNA was as follows: 95°C for 2 min and 40 cycles of 10 s at 95°C, 25 s at 55°C, and 25 s at 72°C. Melting point data and their curves were recorded after qRT-PCR and checked for each well. A control reaction without cDNA was included in each run as a no-template control. Each reaction of real-time PCR was amplified in duplicate.
3.5. Statistical analysis
Efflux pump expression was calculated using the delta cycle threshold method by REST 2009 software (version 2.0.13). The significance level was noted at P ≤ 0.05.
4. Results
4.1. Disc Diffusion and Drop Diffusion
No appropriate or reproducible results were obtained when the effects of NPs with and without antibiotics were tested using the disc diffusion and drop diffusion methods, except for chitosan, which had an increased zone of inhibition against all MDR strains of P. aeruginosa and A. baumannii (Tables 2 and 3). No striking difference was observed between the disc diffusion and drop diffusion methods.
| Synthetic Materials and Concentration, mg/mL | Disc Diffusion Method, mm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P. aeruginosa | A. baumannii | ||||||||
| P1 | P2 | P3 | P4 | PAO1 | A1 | A2 | A3 | ATCC 19606 | |
| AgNPs | |||||||||
| 0.8l | 8 | ||||||||
| 0.4 | 8 | ||||||||
| 0.2 | |||||||||
| 0.1 | |||||||||
| ANC | |||||||||
| 0.3 | 10 | 9 | 9 | ||||||
| 0.1 | 8 | ||||||||
| 0.08 | |||||||||
| ANG | |||||||||
| 1 | 11 | 11 | |||||||
| 0.50 | |||||||||
| 0.25 | - | - | |||||||
| NOCCNPs | |||||||||
| 2.5 | |||||||||
| 1.25 | |||||||||
| 0.6 | |||||||||
| 0.3 | |||||||||
| CNC | |||||||||
| 2.5 | 8 | 11 | 20 | ||||||
| 1.25 | 10 | 16 | |||||||
| 0.6 | 8 | 13 | |||||||
| 0.3 | 9 | ||||||||
| CNG | |||||||||
| 2.5 | 12 | 13 | 15 | 8 | |||||
| 1.25 | 11 | 12 | 13 | ||||||
| 0.6 | 9.5 | 10 | 11.5 | ||||||
| 0.3 | 9 | 9 | 10 | ||||||
| 0.15 | 8 | 8 | 8.5 | ||||||
| NOCC | |||||||||
| 2.5 | |||||||||
| 1.25 | |||||||||
| 0.6 | |||||||||
| 0.3 | |||||||||
| 0.15 | |||||||||
| Chitosan | |||||||||
| 2.5 | 26 | 30 | 33 | 34 | 35 | 24 | 24 | 29 | 30 |
| 1.25 | 23 | 28 | 32 | 32 | 33 | 18 | 19 | 26 | 25 |
| 0.6 | 12.5 | 20 | 27 | 28 | 29 | 13 | 15 | 17 | 22 |
| 0.3 | 9 | 10 | 12 | 18 | 18.5 | 12 | 14 | 12 | 19 |
| 0.15 | 8 | 8 | - | 9 | 12 | 8 | 9 | 10 | 9.5 |
Abbreviation: AgNPs, silver nanoparticles; ANC, silver NPs + ciprofloxacin; ANG, silver NPs + gentamicin; CNC, N,O-carboxymethyl chitosan nanoparticles + ciprofloxacin; CNG, N,O-carboxymethyl chitosan nanoparticles + gentamicin; NOCC, N,O-carboxymethyl chitosan; NOCCNPs, N,O-carboxymethyl chitosan nanoparticles.
| Synthetic Materials and Concentration (mg/ml) | Drop Diffusion Method (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P.Aeruginosa | A.Baumannii | ||||||||
| P1 | P2 | P3 | P4 | PAO1 | A1 | A2 | A3 | ATCC 19606 | |
| AgNPs | |||||||||
| 0.8 | 11 | 10 | 9 | ||||||
| 0.4 | 10 | 8 | 7 | ||||||
| 0.2 | 9 | 6 | |||||||
| 0.1 | |||||||||
| ANC | |||||||||
| 0.3 | |||||||||
| 0.1 | |||||||||
| 0.08 | |||||||||
| ANG | |||||||||
| 1 | 8 | 8 | 8 | 7 | 7.5 | ||||
| 0.50 | 6 | 7 | 6 | 6 | |||||
| 0.25 | 5 | ||||||||
| NOCCNPs | |||||||||
| 2.5 | 8 | ||||||||
| 1.25 | |||||||||
| 0.6 | |||||||||
| 0.3 | |||||||||
| CNC | |||||||||
| 2.5 | 8 | 11.5 | |||||||
| 1.25 | 11 | ||||||||
| 0.6 | |||||||||
| 0.3 | |||||||||
| CNG | |||||||||
| 2.5 | 13 | 11.5 | 14 | 9.5 | |||||
| 1.25 | 12 | 11 | 13 | ||||||
| 0.6 | 10 | 9 | 11.5 | ||||||
| 0.3 | 9 | 8 | 11 | ||||||
| 0.15 | 8 | 7.5 | |||||||
| NOCC | |||||||||
| 2.5 | 10 | 10 | 5 | ||||||
| 1.25 | 5 | ||||||||
| 0.6 | |||||||||
| 0.3 | |||||||||
| 0.15 | |||||||||
| Chitosan | |||||||||
| 2.5 | 9 | 9 | 10 | 11 | 18 | 12 | 12 | 14 | 15 |
| 1.25 | 7 | 7 | 9 | 6 | 15 | 10 | 11 | 10 | 9 |
| 0.6 | 5 | 6 | 8 | 5 | 11 | 9 | 8 | 8 | 8 |
| 0.3 | 5 | 9 | 5 | 7.5 | 7.5 | 7.5 | |||
| 0.15 | 8 | 7 | 7 | 7 | |||||
Abbreviation: AgNPs, silver nanoparticles; ANC, silver NPs + ciprofloxacin; ANG, silver NPs + gentamicin; CNC, N,O-carboxymethyl chitosan nanoparticles + ciprofloxacin; CNG, N,O-carboxymethyl chitosan nanoparticles + gentamicin; NOCC, N,O-carboxymethyl chitosan; NOCCNPs, N,O-carboxymethyl chitosan nanoparticles;.
4.2. MIC and MBC
Table 4 shows the MIC and MBC values of AgNPs, ANC, ANG, NOCCNPs, CNC, CNG, NOCC, and chitosan against the tested bacterial strains. The results indicated that all synthesized NPs had antibacterial activity; however, the potency of chitosan was higher than that of other agents. The MIC of AgNPs against A. baumannii and P. aeruginosa was 0.1 to 0.4 mg/mL and 0.2 mg/mL, respectively. The MIC of ANC against A. baumannii and P. aeruginosa ranged from 0.08 to 0.1 mg/mL. The MIC of ANG against A. baumannii and P. aeruginosa ranged from 0.1 to 1 mg/mL and 0.01 to 0.2 mg/mL, respectively. The MIC of CNG against A. baumannii and P. aeruginosa ranged from 1.2 to 2.5 mg/mL and sensitive to 0.3 mg/ml, respectively. The MIC of CNC against A. baumannii and P. aeruginosa ranged from 0.3 to 1.2 mg/mL and 0.3 to 1.2 mg/ml, respectively. The MIC of chitosan ranged from 0.01 to 0.3 mg/mL against A. baumannii, and it was 0.01 mg/mL against P. aeruginosa.
| Bacteria | MIC/MBC, mg/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| AgNPs | ANC | ANG | CNC | CNG | NOCC NPS | NOCC | Chitosan | |
| P1 | 0.2/0.2 | 0.3/0.3 | 0.1/0.5 | 0.6/0.6 | 0.3/0.3 | 0.3/0.6 | 0.3/0.3 | 0.01/0.06 |
| P2 | 0.2/0.4 | 0.1/0.3 | 0.2/1 | 0.6/0.6 | 0.3/0.3 | 0.3/0.6 | 1.2/1.2 | 0.01/0.06 |
| P3 | 0.2/0.2 | 0.08/0.1 | 0.01/0.06 | 1.2/1.2 | Sensitive | 0.6/1 | 1.2/1.2 | 0.01/0.03 |
| P4 | 0.2/0.8 | 0.1/0.3 | 0.01/0.06 | 0.3/0.6 | Sensitive | 1.2/2.5 | 2.5/2.5 | 0.01/0.03 |
| PAO1 | 0.2/0.2 | 0.08/0.08 | 0.1/0.2 | 0.6/1.2 | 0.01/0.03 | 1.2/2.5 | 1.2.1.2 | 0.01/0.03 |
| A1 | 0.1/0.4 | 0.08/0.3 | 0.1/0.5 | - | 2.5/2.5 | - | 1.2/1.2 | 0.01/0.01 |
| A2 | 0.1/0.8 | 0.08/0.1 | 0.2/0.2 | - | 2.5/2.5 | - | 1.2/1.2 | 0.03/0.03 |
| A3 | 0.8/0.8 | 0.1/0.3 | 1/1 | 1.2/1.2 | 2.5/2.5 | 1.2/- | 1.2/1.2 | 0.03/0.03 |
| ATCC 19606 | 0.4/0.8 | 0.1/0.3 | 0.2/0.5 | 0.3/0.3 | 1.2/1.2 | 1.2/2.5 | 1.2/1.2 | 0.03/0.03 |
4.3. Quantitative RT-PCR Results
The relative expression levels of adeB and MexB genes in A. baumannii and P. aeruginosa with sub-MIC of AgNPs, ANC, ANG, NOCCNPs, CNC, CNG, and chitosan were compared to those of untreated A. baumannii (A1) and P. aeruginosa (PAO1). The results represented the decreased expression of adeB and MexB genes after treating A1 and PAO1 with AgNPs, NOCCNPs, CNC, CNG, and chitosan, while an increased expression of adeB and MexB genes was noted after treating with ANC and ANG, respectively. Figures 1 and 2 show the expression of adeB and MexB after treatment with AgNPs, ANC, ANG, NOCCNPs, CNG, CNC, and chitosan.
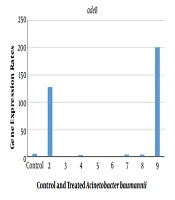
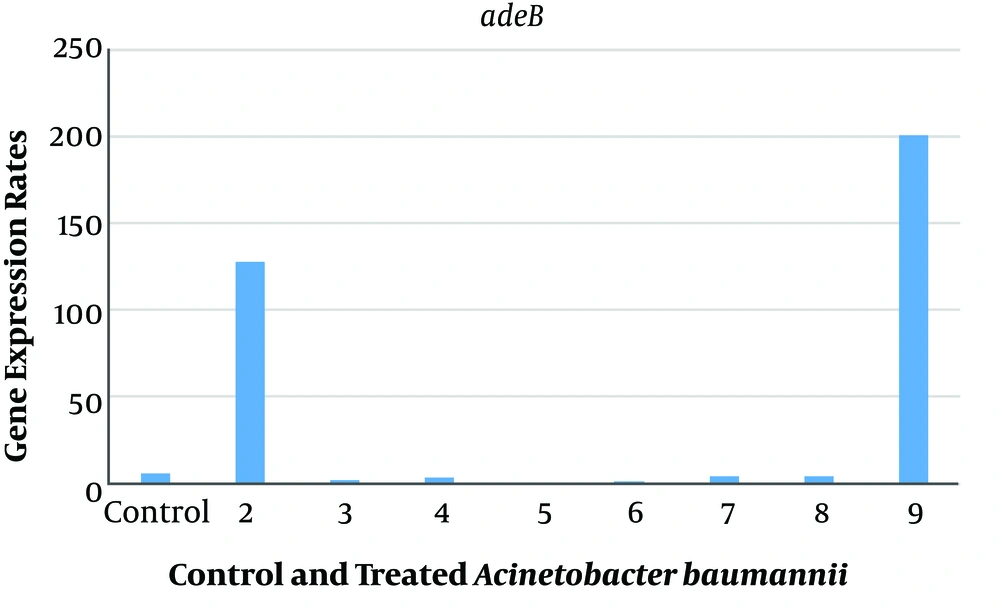
Expression rates of adeB in Acinetobacter baumannii before and after exposure to antibiotics and nanoparticles (NPs). Control, Expression rate of efflux pumps in clinical Acinetobacter baumannii (Ab). 2, expression rate of efflux pumps in Ab exposed to ciprofloxacin; 3, expression rate of efflux pumps in Ab exposed to chitosan; 4, expression rate of efflux pumps in Ab exposed to chitosan nanoparticles with ciprofloxacin (CNC); 5, expression rate of efflux pumps in Ab exposed to chitosan nanoparticles with gentamicin (CNG); 6, expression rate of efflux pumps in Ab exposed to chitosan NPs; 7, expression rate of efflux pumps in Ab exposed to silver nanoparticles (AgNPs); 8, expression rate of efflux pumps in Ab exposed to silver nanoparticles with gentamicin (ANG); 9, expression rate of efflux pumps in Ab exposed to silver nanoparticles with ciprofloxacin (ANC).
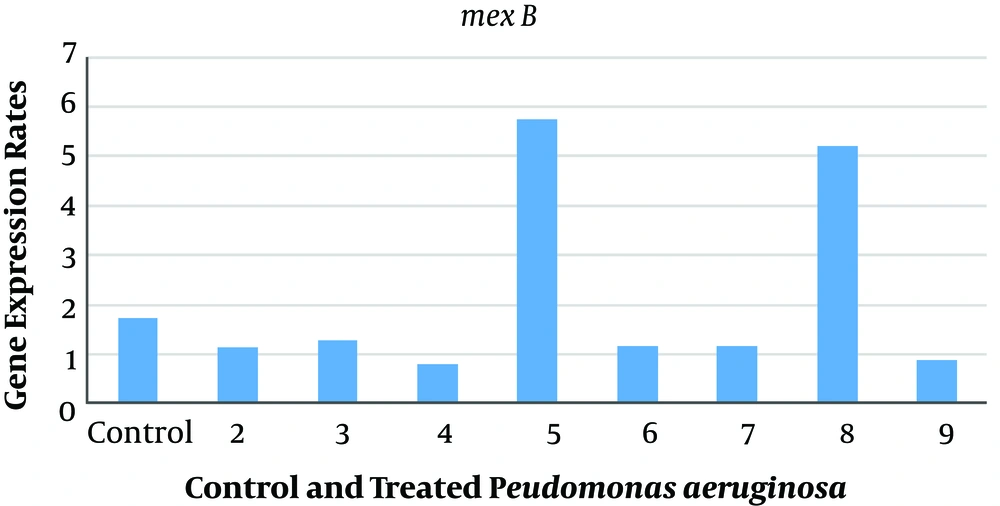
Expression rates of mexB in Pseudomonas aeruginosa PAO1 before and after exposure to antibiotics and nanoparticles (NPs). Control, Expression rate of efflux pumps in Pseudomonas aeruginosa (PAO1); 2, expression rate of efflux pumps in PAO1 exposed to chitosan nanoparticles with gentamicin (CNG); 3, expression rate of efflux pumps in PAO1 exposed to chitosan nanoparticles with ciprofloxacin (CNC); 4, expression rate of efflux pumps in PAO1 exposed to chitosan NPs; 5, expression rate of efflux pumps in PAO1 exposed to silver nanoparticles with gentamicin (ANG); 6, expression rate of efflux pumps in PAO1 exposed to silver nanoparticles (AgNPs); 7, expression rate of efflux pumps in PAO1 exposed to silver nanoparticles with ciprofloxacin (ANC); 8, expression rate of efflux pumps in PAO1 exposed to ciprofloxacin; 9, Expression rate of efflux pumps in PAO1 exposed to chitosan.
An increased expression of adeB was found after clinical A. baumannii strain (A1) was exposed to ANC (32 folds) and ciprofloxacin (20 folds), while a decreased expression of adeB efflux pump was seen after exposure to chitosan (0.4 folds), chitosan NPs (0.3 folds), AgNPs (0.7 folds), CNC (0.6 folds), CNG (0.07 folds), and ANG (0.6 folds).
When the expression level of the mexB gene was studied in P. aeruginosa (PAO1) using real-time PCR, a decreased expression was noted after exposure to chitosan (0.1 folds), CNC (0.7 folds), CNG (0.1 folds), AgNPs (0.3 folds), and ANC (0.007 folds), but an increased expression was observed after exposure to ANG (3 folds) and ciprofloxacin (3 folds).
5. Discussion
The emergence of multidrug-resistant (MDR) bacteria has had an enormous impact on human health worldwide and augmented significant healthcare costs. There is an urgent need for the development of new effective antibacterial strategies, and the use of nanoparticles has proven to be a promising solution to the challenges posed by existing antimicrobials (11).
In the present investigation, AgNPs with an average size of less than 20 nm and NOCCNPs with an average size of 100 nm made the diffusion difficult on the agar medium. Thus, the antibacterial activities of AgNPs, NOCCNPs, ANG, ANC, NOCC, and chitosan against P. aeruginosa and A. baumannii were not the same using the disk agar diffusion and drop diffusion methods. Microdilution broth was the most amenable technique among the three procedures. Unlike other studies (44-46), we observed that the MICs of AgNPs (10 - 20 nm) and its conjugation to antibiotics against P. aeruginosa and A. baumannii were higher (33, 35-37). Martinez-Gutierrez et al. (47) reported the MIC of AgNPs (24 nm) varied from 1.2 to 3.5 μg/mL for clinical antibiotic-resistant E. faecalis, E. coli, S. aureus, P. aeruginosa, and S. maltophilia and 0.4 to 0.7 μg/mL for the ATCC strains of them. Hwang et al. (46) reported the MIC of AgNPs as 0.25 μg/mL for Enterococcus faecium ATCC 19434, 0.5 μg/mL for each of Staphylococcus aureus ATCC 25923, E. coli O157 ATCC 43895, and P. aeruginosa ATCC 27853, and 2 μg/mL for Streptococcus mutants KCTC 3065 and E. coli ATCC 25922. The differences in the MICs of AgNPs against P. aeruginosa and A. baumannii in the present study can be because of the activation of resistance mechanisms such as sil operon, outer membrane variation, and efflux pump activation, as described previously (48, 49). In the current study, AgNPs were stabilized using the carboxyl group of cysteine. The conjugation of antibiotics and nanoparticles was completed by developing peptide bonds between the carboxyl group of nanoparticles and the amine group of ciprofloxacin and/or gentamicin. The MIC range of AgNPs combination with ciprofloxacin and gentamicin (ANC and ANG) did not change in comparison with that of AgNPs. This result shows that the amine group in the antibiotics are very important in the antibacterial effects of the antibiotics.
As mentioned in this study, chitosan showed antibacterial activity against wild-type, MDR, and non-MDR P. aeruginosa, as well as wild-type and MDR A. baumannii by three different methods. In our study, antibacterial activities of NOCCNPs and CNC were seen only against the MDR and ATCC strains of A. baumannii. CNG showed very good antibacterial activity at concentrations of 0.01-0.3 mg/ml against P. aeruginosa, while for A. baumannii, the MIC ranged from 1.2 to 2.5 mg/mL. Dellera et al. (50) demonstrated the MIC of chitosan was 50 μg/ml for S. aureus and 25.8 μg/mL for E. coli. Sadeghi et al. (51) reported quite high MIC values (1000 μg/ml) of chitosan for S. aureus. The MIC of chitosan against A. baumannii and P. aeruginosa in our study was 10 - 30 μg/mL, which was lower than the MIC of chitosan against S. aureus; this can be explained by the more susceptibility of Gram-negative bacteria than Gram-positive ones against chitosan.
In another research study by Saito et al. (52), the antibacterial activity of conjugated chitosan with lysozyme was evaluated against A.baumannii and P. aeruginosa, and it was found as 400 and 200 μg/mL in the MDR and PAO1 strains of P. aeruginosa, respectively. While in our study, the MIC of conjugated chitosan with gentamicin (CNG) was 300 μg/mL for MDR P. aeruginosa, 10 μg/mL for PAO1, and without any growth in non-MDR P. aeruginosa. This shows that CNG in our study had a high antibacterial effect on non-MDR P. aeruginosa, MDR P. aeruginosa, and PAO1 when compared to conjugated chitosan with lysozyme.
In the present investigation, carboxymethyl groups were substituted in some amino groups of the glucosamine units of chitosan by using monochloroacetic acid in an alkaline medium, and then they were converted to nanoparticles (NOCCNPs) using sodium Tripolyphosphate (TPP). Antibiotics and nanoparticles were combined using covalence bonds between the amine groups of ciprofloxacin and/or gentamicin and the carboxyl group of NOCCNPs. Although the MIC of chitosan, NOCCNPs, and its combination with antibiotics (CNC and CNG) showed acceptable antibacterial activities against P. aeruginosa and A. baumannii, chitosan was superior in the free form with many amine groups and had lower MIC when compared to CNC and CNG. Compatible with our research, Sadeghi et al. (51) also demonstrated that the antibacterial activity of chitosan, N-trimethyl chitosan (TMC), and N-diethyl methyl chitosan (DEMC) against S. aureus was higher than that of its combined NP form.
In our study, the adeB and mexB gene expressions were studied before and after the treatment of A. baumannii ATCC 19606, while the PAO1 wild-type strain of P. aeruginosa was chosen as control. To avoid any differences in efflux pump gene expression that may exist among different strains, we used only one strain of A. baumannii and P. aeruginosa and assessed any changes in their expressions. The downregulation of adeB and mexB was seen after treatment with chitosan, NOCCNPs, CNC, CNG, and AgNPs. On the other hand, the upregulation of adeB and mexB was noticed after treatment with ANC and ANG, respectively. Other researchers showed that the MexAB efflux pump in P. aeruginosa can efflux silver nanoparticles outside the bacterial cell. But, in the presence of antibiotics like aztreonam or chloramphenicol, the efflux pump cannot work properly because of extreme damage in the cell wall; thereby, nanoparticles and antibiotics accumulate in the bacterial cell at high concentrations (53, 54). Similar to this finding, we found the downregulation of adeB and mexB after treatment of bacteria with chitosan and its nanoparticles combined with antibiotics and the upregulation of adeB and mexB was a remarkable feature noticed after treatment with ANC and ANG, respectively.
5.1. Conclusions
Though the antibacterial activity of chitosan can be evaluated by many phenotypic methods, microdilution broth was an appropriate method for testing the antibacterial activity of silver and chitosan nanoparticles alone or in combination with antibiotics. In addition to its antimicrobial properties, the results showed that chitosan with medium molecular weight, its combinations with antibiotics (CNC and CNG), and AgNPs all had anti-efflux pump activity against A. baumannii and P. aeruginosa. Thus, they may serve as Efflux Pump Inhibitors (EPI) in laboratory research.