1. Background
Hepatitis A virus (HAV) infection is a common cause of acute liver disease (1). Tens of millions of people are annually infected with HAV around the world. The disease is mainly transmitted through the fecal-oral route, direct contact with infected patients, and consuming contaminated water and food (2). HAV seroprevalence among Iranian populations varies from 90 to 99% in adults (3, 4) and 51 to 85% in children (5, 6). Although the disease is generally a self-limiting condition, it can also be a life-threat with 0.3% - 0.6% to 1.8% mortality rate among people over the age of 50 years (7).
The mechanisms of liver damage by HAV are not well known, and limited information is available on the immunological effects of HAV infection (8). Most recently, the role of cytotoxicity by CD 8+ T lymphocytes has been highlighted in acute HAV infection (9). HAV seems to infect only proliferative hepatocytes; however, it may also penetrate into gastrointestinal cells. Despite its high affinity for living cells, HAV represents no cytopathic effects against them, and extra-hepatic manifestations of HAV infection are unusual. Nevertheless, there are reports of detrimental effects on the cardiovascular system, bone marrow, kidney, as well as biliary and pancreatic systems (9-12).
Assessment of liver enzymes is the main diagnostic and monitoring tool for various types of liver diseases. Accordingly, asymptomatic viral hepatic infections may only be detectable by elevated aminotransferases (13). The most common evaluated hepatic enzymes are alanine amino transaminase (ALT) and aspartate amino transaminase (AST). In most cases, including acute HAV infection, the levels of these liver enzymes are mildly and temporarily elevated. Unlike other types of viral hepatitis infections, HAV does not cause long-term damage to the liver or progress to chronic conditions (14).
Vitamin E is an important micronutrient that the body itself cannot produce, so it is merely obtained from diet (15). Vitamin E is a fat-soluble and antioxidant compound that rapidly reacts with organic free radicals to protect cells against oxidation and degradation of their organelles. Vitamin E is the first line of defense against lipid peroxidation and also important for the function of immune cells (16). Vitamin E has been reported to improve liver inflammation and fibrosis by regulating inflammatory signaling pathways and decreasing the expression of TGF-β1, TNF-α, IL-6, and other inflammatory cytokines (17). There are reports on the positive and safe effects of this anti-oxidative vitamin in patients with hepatitis C (HCV) (18, 19) and chronic hepatitis B (HBV) (20, 21) infections, as well as nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease (NAFLD) (22).
2. Objectives
There were no studies on the potential beneficial effects of vitamin E in acute pediatric HAV infection. In the present study, we investigated the effect of vitamin E on the improvement of hepatic enzymes in children with acute hepatitis A.
3. Methods
3.1. Patients
This was a randomized clinical trial in children with acute HAV infection. The study had the approval of the local Ethics Committee of Zabol University of Medical Sciences (code Zbmu.1.REC.1396.63). Overall, 142 patients with acute HAV infection admitted to the Amir-Al-Momenin Hospital of Zabol were recruited from February 2016 to August 2017. The study has been registered in The Iranian Registry of Clinical Trials (code: IRCT20171205037752N1).
3.2. Inclusion Criteria
These included children with positive anti HAV with signs of coagulopathy and/or encephalopathy who were inpatients in our hospital. These were children who were hospitalized in our ward and represented signs and symptoms of coagulopathy and/or encephalopathy based on coagulation tests and electroencephalogram as well as clinical examinations by a pediatric hepatologist and neurologist.
3.3. Exclusion Criteria
These included anti HAV positivity for up to 6 months (i.e. chronic HAV infection), abdominal pain (to exclude those that may have concomitant celiac disease), discolored urine and/or stool samples, and being under treatment with any medication.
3.4. Randomization and Blinding
After obtaining informed consent from patients’ parents, a checklist was completed to obtain demographic characteristics. Then, eligible patients were randomly (www.randomizer.org) divided into either intervention or control groups. Patients in the intervention group received 400 milligrams of vitamin E daily consumed as a single dose along with fatty foods. The control group did not receive any treatment. This study was a single-blinded trial in which the researchers who measured liver function tests were not aware of the patients’ group allocation.
3.5. Liver Enzyme Monitoring
Liver enzymes AST and ALT were checked at admission, after two weeks, one month, two months, three months, four months, and six months of therapy initiation. During these times, the clinical condition of the patients was monitored by the researchers.
3.6. Statistical Analysis
Statistical methods were performed in SPSS version 19 software. The Shapiro-Wilk test was recruited to assess normal distribution. Comparisons of liver enzymes between groups were done using the Mann-Whitney U-test at each time point. The within comparisons of liver enzymes during the time-course of study was conducted using two-way repeated-measures ANOVA test. A P-value < 0.05 was considered as statistically significant.
4. Results
In this study, 142 children with acute HAV infection were recruited. These were randomly allocated into control (n = 71) or vitamin E treated (n = 71) groups. Male participants constituted 36 (50.7%) and 35 (49.3%) in the control and intervention groups, respectively. The mean ages of the patients were 8.4 ± 2.5 and 9 ± 4.3 in the control and intervention groups, respectively (Table 1).
| Variables | Study Groups | P | |
|---|---|---|---|
| Control (N = 71) | Vit E (N = 71) | ||
| Age, y | 8.4 ± 2.5 | 9 ± 4.3 | 0.1 |
| WBC, × 103/µL | 7835.3 ± 3254.2 | 7550.7 ± 3008.6 | 0.5 |
| Hemoglobin, g/dL | 11.6 ± 1.6 | 11.9 ± 1.3 | 0.2 |
| Platelet, × 103/µL | 354.5 ± 104.1 | 316.1 ± 97.5 | 0.02 |
| Albumin, g/dL | 4.35 ± 0.74 | 4.34 ± 0.51 | 0.9 |
| Total Bilirubin, mg/dL | 4.90 ± 4.93 | 3.72 ± 3.18 | 0.09 |
| Direct bilirubin, mg/dL | 2.79 ± 4.27 | 1.87 ± 2.22 | 0.1 |
| Total serum protein, g/dL | 6.90 ± 0.90 | 7.12 ± 0.85 | 0.1 |
aValues are expressed as mean ± SD.
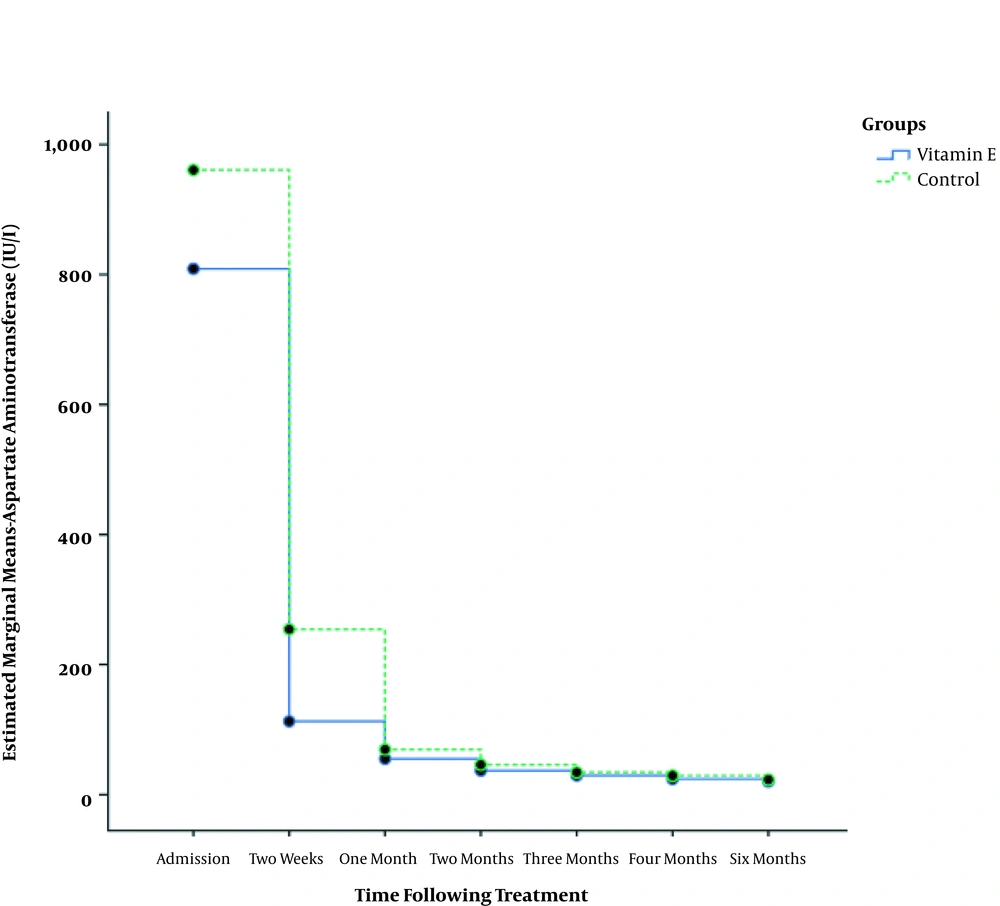
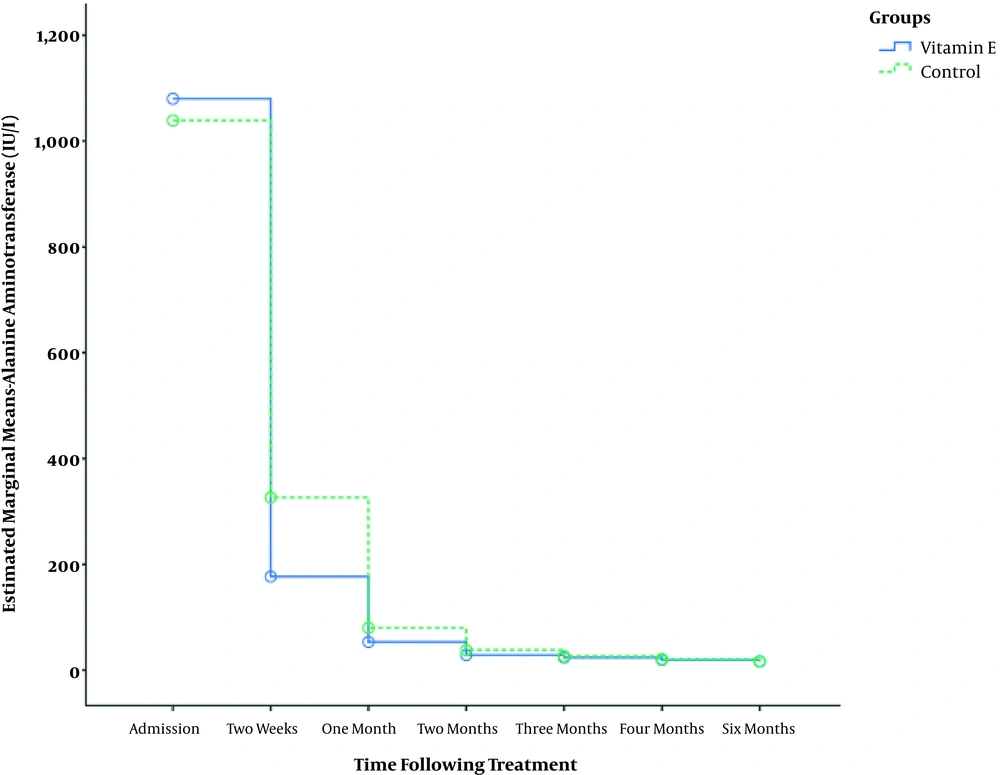
Table 2 shows that in both the study groups, AST level significantly decreased over time (P < 0.001). Comparing the two groups, the AST level was found to be significantly lower in the vitamin E supplemented group at 1, 2, 3, 4, and 6 months following therapy (Figure 1). Also, ALT level significantly decreased in both groups during the six months of the study (P < 0.001); however, no significant difference was detected between the groups except for two months post-treatment (P = 0.01, Table 3 and Figure 2).
| Variables | Aspartate Aminotransferase Level, IU/L | P | |
|---|---|---|---|
| Control (N = 71) | Vit E (N = 71) | ||
| At admission | 960.8 ± 1180 | 808.7 ± 857 | 0.47 |
| Two weeks | 254.2 ± 576 | 112.7 ± 142 | 0.23 |
| One month | 69.4 ± 48 | 55.1 ± 34 | 0.01 |
| Two months | 46 ± 22 | 36.9 ± 12 | 0.002 |
| Three months | 34.2 ± 11 | 29.3 ± 8 | 0.005 |
| Four months | 29.2 ± 11 | 24 ± 7 | 0.002 |
| Six months | 23.1 ± 7 | 20 ± 6 | 0.002 |
| P | < 0.001 | < 0.001 | |
aValues are expressed as mean ± SD.
| Variables | Alanine Aminotransferase Level, IU/L | P | |
|---|---|---|---|
| Control (N = 71) | Vit E (N = 71) | ||
| At admission | 1038.8 ± 926 | 1080 ± 1038 | 0.80 |
| Two weeks | 326.5 ± 818 | 176.8 ± 247 | 0.14 |
| One month | 79.9 ± 112 | 53.3 ± 60 | |
| Two months | 38.1 ± 30 | 28.6 ± 13 | 0.01 |
| Three months | 26.6 ± 12 | 23.8 ± 10 | 0.14 |
| Four months | 20.8 ± 7 | 19.5 ± 6 | 0.25 |
| Six months | 16.6 ± 5 | 16.5 ± 7 | 0.88 |
| P | < 0.001 | < 0.001 | |
aValues are expressed as mean ± SD.
5. Discussion
Viral hepatitis is a widespread inflammatory disease that can inflict acute or chronic (or both) liver injuries (23). In the present study, although AST and ALT significantly decreased in both vitamin E-supplemented and control groups over the six-month period of the study, we observed that the children who received vitamin E had significantly lower AST levels at 1, 2, 3, 4, and 6 months and ALT level at 2 months post-therapy. This showed that vitamin E accelerated the normalization of liver enzymes, especially AST, in these patients, and therefore it can be used as a supplementary compound to induce a faster recovery in children with acute HAV infection.
Our results were in agreement with the results of Sanyal et al. (24) in 2010, stating that vitamin E was superior to placebo in treating people with nonalcoholic liver disease without diabetes. One study on 17 patients with chronic HCV infection showed that vitamin E supplementation significantly lowered ALT after 1, 2, and 3 months, especially in patients with high baseline ALT (> 70 IU/L), while this effect was not observed in those who had a primary ALT level of < 70 IU/L (18). In another study on patients with chronic HBV infection, ALT normalization was noted in 47% of vitamin E-supplemented and 6% of the control group (P = 0.01) (21). Furthermore, vitamin E also induced a significantly higher molecular response (HBV-DNA negativity) in patients with chronic HBV (21). In the patients infected with HCV (genotype 3) infection, administration of vitamin E for three months significantly reduced ALT in 57.8% of patients in comparison with 29.4% in the control group (19). A meta-analysis study also confirmed the beneficial effects of vitamin E in normalizing AST and ALT in patients NAFLD, NASH, and chronic HCV infection (22). In the study of Khajeh Jahromi et al. (25) who compared the therapeutic effects of three drugs: melatonin, metformin and vitamin E in patients with NAFLD, it was found that vitamin E supplementation significantly reduced the liver damage (i.e. decreased levels of AST and ALT enzymes) in these patients. In another study on the effect of supplementation of vitamin E on liver enzymes in patients with NASH, there was a significant reduction in the levels of AST and ALT in the group receiving vitamin E supplements (24). Yakaryilmaz et al. (26) also found that hepatic enzymes were significantly improved following vitamin E intake in patients with NASH. Furthermore, Madan et al. (27) also observed that a diet containing vitamin E was effective in normalizing ALT levels in patients with plain fatty liver.
These effects are supposed to be related to the hepatoprotective effects of vitamin E against the detrimental effects of oxidative stress (18). Vitamin E has also been shown to enhance functional indices of T lymphocytes and antibody response to the pathogens, which may be in part be involved in its observed hepatoprotective effects (28). In accordance, vitamin E was also effective in preserving the viability of mice-derived liver cells against silver nanoparticle-induced cytotoxicity (29). Accordingly, vitamin E supplementation was also effective in the reduction of AST and ALT in patients suffering from hepatitis C virus (HCV) (30) and hepatitis B virus (HBV) (21) infections, at least partly through augmenting the host’s immune responses against the infection (21). The disease’s nature may be another important factor influencing the health effects of vitamin E. In line, Ji et al. (22) reported that vitamin E administration was effective in lowering hepatic enzymes in patients with NASH and chronic HCV infection, but not NAFLD. Vitamin E can scavenge free radical species from hepatocytes and therefore alleviate oxidative stress-induced cellular damage in the liver (13, 23). This is important knowing that oxidant species can augment the production of inflammatory cytokines (such as IL-1, IL-6, TNFα, and TGF-β1) and subsequently lead to a persisted inflammatory status in the liver (31, 32). Finally, vitamin E can prevent liver inflammation and fibrosis by modulating multiple cellular processes, cell signaling pathways, and gene expression patterns (31).
Contrary to the above-mentioned, there are also reports conflicting the beneficial roles of vitamin E in improving hepatic liver enzymes. In opposition to these findings, in a study on children with immunotolerant HBV infection, Dikici et al. (33) did not find any significant difference comparing ALT levels between patients who were treated with vitamin E and those who were not at neither three nor nine months after treatment. Lavine et al. (34) examined the effects of vitamin E and metformin against placebo in the treatment of NAFLD, and stated that neither vitamin E nor metformin could significantly impact the levels of aminotransferases compared to placebo. In another study by Zelber-Sagi et al. (35) that evaluated the effects of exercise, nutrition, and vitamin E supplement in patients with NAFLD, vitamin E did not exert any influential impact on the inflammatory activity or ALT levels compared with placebo. In a study by Kugelmas et al. (36), vitamin E also showed no effects on reducing the level of liver enzymes in patients with NASH. In line, Mezey et al. (37) also described that although vitamin E significantly reduced serum hyaluronic acid levels, but the supplement had no beneficial effects on bilirubin and liver enzymes in alcoholic hepatitis patients. Therefore, different studies indicate significant variabilities in the response of the liver cells to vitamin E in different hepatic disorders, which highlights the necessity of performing more standard clinical trials and meta-analyses on this issue.
5.1. Conclusions
We hereby implicated that vitamin E can be an effective complement to accelerate restoring hepatic function in children suffering from acute HAV infection. Nevertheless, limited information is available on the immunological aspects of HAV infection that can have significant impacts on the functions of vitamin E. HAV does not usually affect liver function for a long time period, with more than 99% of patients recovering. In symptomatic patients, one needs to rest until the immune system gradually overcomes the infection and restores normal hepatic function. Nonetheless, accelerating the recovery period in acute HAV infection, especially in susceptible populations such as children, may be of crucial importance.