1. Introduction
The coronavirus disease (Covid-19) outbreak is affecting 195 countries and territories all around the world. By March 29, 2020, 737000 people were infected and 35000 died worldwide (1). Covid-19 typically causes flu-like symptoms, including fever and cough. In some patients, particularly older people and those with chronic diseases, the virus causes pneumonia, with chest tightness, chest pain, and shortness of breath (2, 3). To date, no effective medication is recommended to treat SARS-CoV-2 infection, and the drugs introduced are still in the clinical trial phase. Tocilizumab and Remdisivir are possible drugs to treat and improve the symptoms of the diseases (4, 5).
Tocilizumab, also known as atlizumab and Actemra, is an immunosuppressive humanized monoclonal antibody drug (4). Although its exact mechanism to protect the respiratory system against the Covid-19 is not yet elucidated, some studies reported that Tocilizumab’s effects on interleukin six receptor can inhibit inflammatory reactions and through which improves the oxygen saturation (5, 6). Remdesivir is a monophosphoramidate prodrug and an adenosine analog with a development code GS-5734 that has a broad-spectrum antiviral. since 2017, it was synthesized and developed by Gilead as a treatment for Ebola virus infection (7). Remdesivir is metabolized into its active form, GS-441524, that obscures viral RNA polymerase and evades proofreading by viral exonuclease, causing a decrease in viral RNA production. In-vitro studies reported that Remdesivir can inhibit coronaviruses, such as SARS-COV and MERS-COV replication (8, 9).
As combination therapy with antiviral and anti-inflammatory may be an effective treatment to reduce inflammation and viral infectivity, we prescribed a combination of Remdisivir and Tocilizumab to treat three critical patients with Covid-19. For all three patients, Remdisivir 200 mg stat and 100 mg per day were administered for five days, and Tocilizumab was administered three-dose 400 mg infusion in 60 minutes every other day. In general, combination therapy was performed within 5 days.
2. Case Presentation
2.1. Case 1
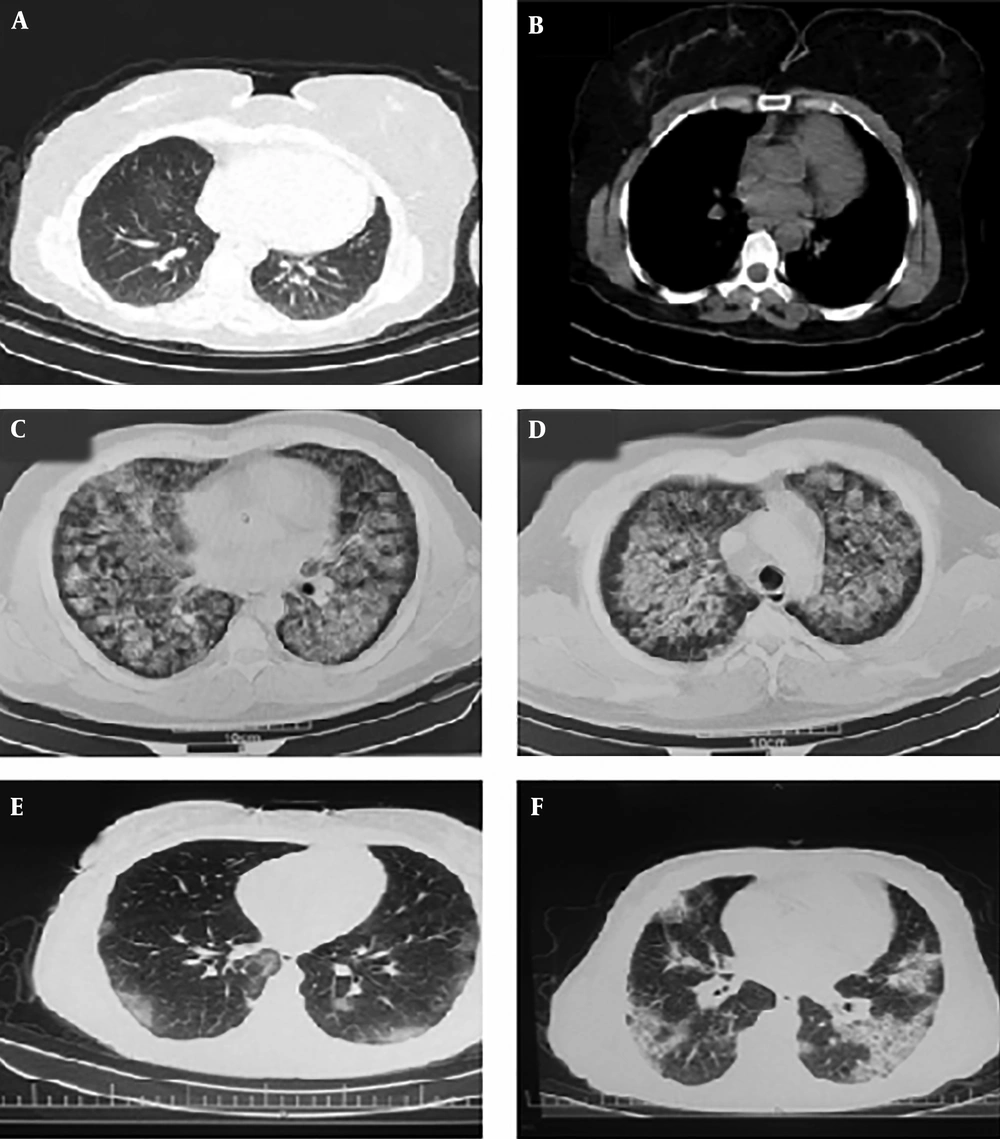
The first case was a 55 years women with 155 cm height and 105 kg weight who was living in Mazandaran province. Her primary symptoms were fever, shivering, and anorexia. Besides, she had severe myalgia, cough, arthralgia, fever, and dyspnea for three days before the admission. On March 16 2020, she was hospitalized. Primary evaluation and medical history revealed a history of hypertension and diabetes mellitus. In auscultation of lungs, sounds were heard. Her main problems were nausea and vomiting. She was first treated with routine oral treatment, but her health status was deteriorated. Chest computerized tomography (CT) indicated signs of the pleural effusions (Figure 1A). After confirmation of Covid-19 infection with rRT-PCR and Due to the persistent hyperpyrexia, she was transferred to an isolation room in the Intensive Care Unit (ICU) on March 19, 2020. When her SPO2 reached below 70% the intubation was begun. On March 20 2020, patient cardiac arrhythmias led to a cardiac arrest. Immediately, the CPR was begun, and after 10 minutes, resuscitation returned.
2.2. Case 2
The second case was a 58 years man with 178 cm in height and 78 kg weight. On March 15, 2020, he was transferred to the emergency department with fever, shivering, and anorexia. On admission, his vital signs were stable. However, the high fever, shivering, and dyspnea were continuing. The patient had no history of any illness or drug use. The CT indicated signs of the pleural effusions (Figure 1E). The swab specimen was tested positive for SARS-CoV-2 by real-time reverse transcriptase-polymerase-chain-reaction (rRT-PCR) on March 16, 2020. The patient was healthy before this outbreak. He was transferred to ICU for further treatment. But despite treatment with the aforementioned drugs and providing special care in the ICU, he died on March 27, 2020.
2.3. Case 3
The third case was an 83 years old man with 170 cm height and 79 kg weight who was living in the Mazandaran province. He had dyspnea from 10 days ago. On March 24 2020, the patient transferred to the hospital with severe symptoms such as dyspnea, cough, constipation, and loss of consciousness. He had a history of CABG and urinary incontinence. In clinical examination, rales heard in base of lungs. The CT indicated signs of the pleural effusions (Figure 1E). The triage level based on the ESI scale was 2 and the patient transferred to the ICU. Infection with Covid-19 was confirmed on March 25 after performing swab specimen and rRT-PCR. A 4 days combination therapy was administered, but he died on March 24 2020.
CT scans of three patients are shown in Figure 1B and F. Clinical laboratory data, vital signs, and arterial blood gas (ABG) are described in Tables 1 and 2, respectively.
| Patient Parameter | Case 1 | Case 2 | Case 3 | |||
|---|---|---|---|---|---|---|
| On Set | 5th Day | On Set | 5th Day | On Set | 5th Day | |
| Lymphosite, /µL | 1800 (20.1) | 1300 (20.6) | 1500 (23.5) | 700 (10.8) | 500 (12.4) | 600 (8.2) |
| Neutrophil , /µL | 5600 (63.5) | 4900 (76.4) | 4400 (68.8) | 24700 (86.9) | 3600 (84.9) | 6500 (90.6) |
| WBC, /µL | 8800 | 6400 | 6400 | 28500 | 4200 | 7200 |
| RBC, /µL | 3100000 | 3850000 | 4410000 | 3810000 | 4020000 | 3990000 |
| PLT, /µL | 130000 | 100000 | 140000 | 15000 | 74000 | 51000 |
| LDH, IU/I | 881 | 900 | 200 | 220 | 2088 | 2100 |
| FBS, mg/dL | 131 | 128 | 149 | 129 | 74 | 84 |
| Na, mEq/L | 142 | 142 | 142 | 145 | 146 | 145 |
| K, mEq/L | 3.9 | 4 | 4 | 4.2 | 4.1 | 4 |
| BUN, mg/dL | 48 | 49 | 30 | 98 | 50 | 80 |
| Cr, mg/dL | 0.8 | 1 | 1 | 1.2 | 1.3 | 1.5 |
| HCT, % | 29.8 | 35.8 | 37.2 | 36 | 34.2 | 33 |
| Hb, g/dL | 9.1 | 11.6 | 12.8 | 11 | 11.2 | 11 |
| PT | 13.6 | 15 | 15.7 | 18 | 17.3 | 17.5 |
| PTT | 45 | 43 | 30 | 56 | 36 | 40 |
| INR | 1.22 | 1.45 | 1.52 | 1.88 | 1.77 | 1.6 |
| AST, Umol/L | 27 | 29 | 28 | 29 | 75 | 72 |
| ALT, Umol/L | 18 | 20 | 21 | 20 | 22 | 24 |
| ALP, U/L | 161 | 170 | 168 | 171 | 180 | 183 |
aValues are expressed as No. (%).
| Vital Sign and ABG | Case 1 | Case 2 | Case 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On Set | 2nd Day | 3rd Day | 4th Day | 5th Day | On Set | 2nd Day | 3rd Day | 4th Day | 5th Day | On Set | 2nd Day | 3rd Day | 4th Day | 5th Day | |
| SPO2, % | 58.4 | 60 | 81 | 95 | expired | 83 | 78 | 85 | 89 | 86 | 95 | 91 | 89 | 86 | 93 |
| HR, beat/min | 95 | 100 | 96 | 85 | expired | 98 | 100 | 105 | 101 | 115 | 94 | 91 | 94 | 110 | 83 |
| RR | 28 | 24 | 23 | 20 | expired | 40 | 25 | 22 | 18 | 17 | 19 | 21 | 28 | 25 | 16 |
| T | 37.8 | 37.5 | 36.9 | 36.9 | expired | 37.1 | 36.8 | 36.9 | 36.8 | 37.5 | 36.8 | 36.8 | 36.9 | 36.9 | 37 |
| SBP, mmHg | 145 | 148 | 130 | 150 | expired | 156 | 148 | 112 | 129 | 112 | 121 | 122 | 92 | 110 | 140 |
| DBP, mmHg | 78 | 80 | 85 | 87 | expired | 80 | 90 | 82 | 80 | 70 | 81 | 82 | 61 | 60 | 81 |
| PH | 7.41 | 7.28 | 7.43 | 7.5 | expired | 7.25 | 7.39 | 7.36 | 7.32 | 7.37 | 7.15 | 7.20 | 7.25 | 7.10 | 7.45 |
| PaO2,mmHg | 30 | 30 | 45 | 66 | expired | 22 | 44 | 31 | 52 | 86 | 25 | 30 | 45 | 55 | 63.5 |
| PaCO2,mmHg | 39.8 | 64 | 46.2 | 46.9 | expired | 57.8 | 42.7 | 53.4 | 54.7 | 55.8 | 55 | 54 | 52 | 57 | 27.8 |
| HCO3 | 25.6 | 30.8 | 30.6 | 36 | expired | 25.8 | 25.7 | 30.3 | 27.9 | 32.3 | 22 | 25 | 28.3 | 30 | 24 |
3. Discussion
The SARS-CoV-2 emerged in December 2019 and spread rapidly worldwide, particularly in China, Japan, South Korea, and many other countries (9-11). Iran is also affected by this fatal virus, and many people are infected (6). All around the world, researchers are trying to find a potential therapeutic agent (10). A lack of proper treatment for the virus has raised concerns among the public and officials (11).
On January 25, 2020, a joint research team of the Shanghai Institute of Materia Medica and Shanghai Tech University performed investigations on silicon and an enzyme activity test. They reported 30 agents with potential antiviral activity against SARS-CoV-2 that one of them was Remdesivir (12). Using the analysis of protease and RNA polymerase docking, Chang et al. (10) demonstrated that among the potential therapeutic agents for Covid-19, Remdesivir had limited toxicity in clinical practices, and recommend its administration in treating Covid-19 patients. It’s well-documented that the host immune response is an important factor leading to coping with life-threatening ARDS in Covid-19 patients (13). Tocilizumab may have a positive effect on improving the immune damaging, lung functional injuries, and arterial oxygen saturation (14).
Due to the nature of the disease and its inflammatory effects on lung tissues, combined anti-inflammatory and antiviral treatments for Covid-19 infections is suggested as an effective approach (12, 15).
Based on the presented cases, combined therapy of Tocilizumab and Remdesivir may not be an effective treatment for all patients infected with Covid-19. For the first case, the combined treatment improved the health status of the patient, and she was discharged from the ICU. But for other cases, the combined treatment had no significant effect and they died.
3.1. Conclusions
Recently combined anti-inflammatory and antiviral treatments for Covid-19 infections is suggested as an effective approach to treat patients. Researchers are investigating different drugs to find an effective therapeutic solution to inhibit the SARS-COV2 virus and to reduce or decelerate its effect. Combined therapy with Tocilizumab and Remdesivir in three cases showed different results. If prescribed for the right people, with the right dose, and at the right time, combining these two medications may be effective. To understand the efficacy of these drugs, further preclinical and clinical studies should be performed.