1. Background
Malaria is one of the important infectious blood diseases caused by the protozoan parasite of the genus Plasmodium and transmitted by female Anopheles mosquito bites (1, 2). To date, five species of Plasmodium have been reported to cause human malaria, among which Plasmodium falciparum is considered a malignant species and linked to the highest rate of deaths from malaria. Another species is Plasmodium vivax accounting for the most positive cases of malaria in the world. However, this species is associated with a lower incidence of malaria deaths. Two species of Plasmodium ovale and Plasmodium malariae generally have been reported to cause a moderate form of the disease. The last species is Plasmodium knowlesi that has been responsible for sporadic cases of malaria in southeast Asia in recent years (3).
Globally, 229 million cases of malaria have been reported by the World Health Organization (WHO) in 2020 of which, 409,000 died. Also, more than 87 countries have reported malaria transmission (4). The impact of malaria disease on public health and health promotion criteria located malaria in the focus of international organizations related to the health of societies. These organizations approved the conclusive programs of control, elimination, and eradication of malaria (5).
In recent years, there has been a significant decline in the number of positive cases of malaria in Iran. According to WHO, Iran is in the process of eliminating malaria. The first phase of the malaria elimination program (2010 - 2015), with the support of WHO, was successfully implemented in Iran, and its second phase (2016 - 2020) has been started since 2016 and is running now (3). Given that no local transmission of malaria was observed in Iran during 2018 - 2019 according to the WHO report, and considering the appropriate status of the malaria elimination plan, obtaining a certificate of malaria elimination is now possible (4, 6).
Asymptomatic malaria is a major challenge to the malaria elimination plan. It causes a reservoir, which can contribute to the ongoing transmission of disease (7). The reservoir of asymptomatic malaria has been investigated in some studies in Iran (8-16) and other countries (17-25). The results reported in these studies are inconsistent in terms of the presence of positive cases. A malaria elimination plan is currently being followed in Hormozgan Province. The robust malaria surveillance system with appropriate active case findings can play an important role in the malaria elimination program (26).
2. Objectives
The main objectives of this research were to determine the presence and prevalence of asymptomatic malaria cases and monitor asymptomatic parasitic reservoirs in Jask District, Hormozgan Province. According to the results of previous studies showing that routine diagnostic techniques (microscopy and RDT) do not make enough sensitivity to diagnose asymptomatic malaria cases, a molecular assay as a robust and sensitive method was added in this study. The findings of the present study could support the successful implementation of the malaria elimination program in Hormozgan and Iran by providing basic information and identifying challenges.
3. Methods
3.1. Study Area
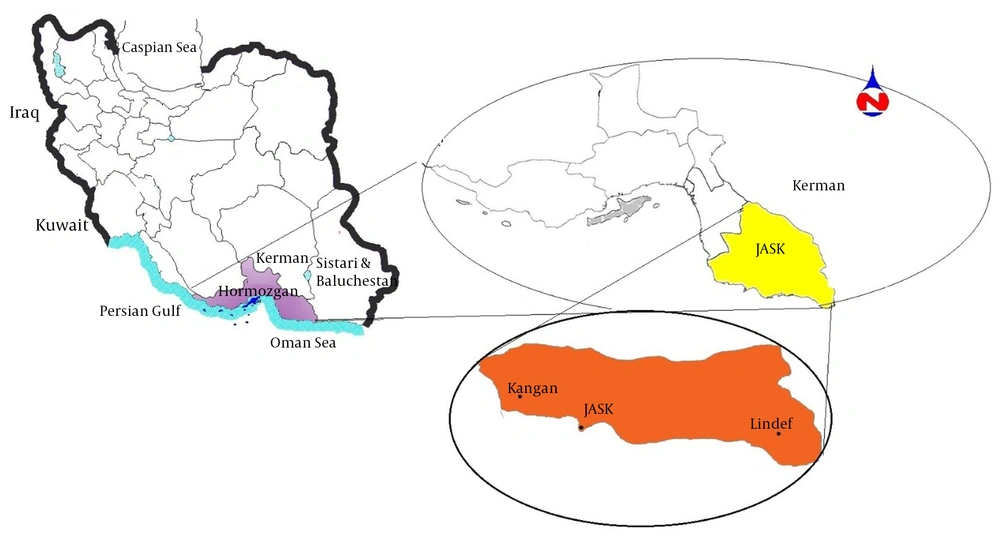
This study was performed in Jask County as an endemic area of malaria in Hormozgan Province. Jask County with an area of 16136.2 km2, located at a longitude of 57°10’ - 59°16’E and latitude of 25°23’ - 26°13’N, is in the southeast of Hormozgan Province. Jask County is bounded on the east and northeast by Sistan and Baluchestan Province. The south and southwest of the county are adjacent to the waters of the Oman Sea and the Strait of Hormuz (Figure 1) (http://hormozgan.rmto.ir/English/Pages/Introduction.aspx). According to the 2017 census, the population of Jask County was about 58,884. The city’s climate is described as hot and dry in summer and temperate in winter, with a relative humidity of more than 50% across the coastal areas (Figure 1). Jask County is one of the malarious areas of Hormozgan Province in which malaria has been reported in recent years. Due to proximity to Sistan and Baluchistan Province, the arrival of foreigners, population displacement, and appropriate weather conditions, Jask County is susceptible to malaria transmission almost year-round. According to the WHO benchmark, Jask is now in the malaria elimination phase (API < 1/1000 per year).
3.2. Study Population and Inclusion Criteria
This cross-sectional study aimed to evaluate and monitor asymptomatic cases in the Jask District. A total of 230 (asymptomatic residents 124 females and 86 males) were randomly selected, and their blood samples (3 mL) were taken to assess Plasmodium infection using microscopic, RDT, and molecular (18ssrRNA) methods (Table 1).
| Study Subjects | Age Group, y | Gender | ||||
|---|---|---|---|---|---|---|
| Group | < 15 | 15 - 30 | 30 - 45 | > 45 | Female | Male |
| Subject, No. (%) | 29 (12.6) | 154 (67) | 37 (16) | 10 (4.4) | 126 (54.8) | 104 (45.2) |
| Total | 230 (100) | |||||
The purpose and stages of the study were explained to all participants/parents, and written informed consent was obtained. After interviewing and recording the demographic data (name, age, sex, job, immigration history, and malaria history), the presence or absence of clinical malaria signs was evaluated. The current study included those participants who had no clinical symptoms of malaria, and excluded those who had used anti-malarial drugs in the past month and traveled to other malaria-endemic areas in the past three months. Pregnant women and people under four or over 60 years old were also excluded from the study.
3.2.1. Ethical Approval
The project was done as per the ethical principles and the national norms and standards for conducting medical research in Iran. The study was approved by the Iran National Committee for Ethics in Biomedical Research (approval ID: IR.HUMS.REC.92-4-1).
3.3. Diagnostic Tests
3.3.1. Microscopic Examination (Direct Examination Method)
The microscopic or direct diagnosis method of malaria was carried out according to the guidelines approved by the WHO (27). The microscopic technique is the common and gold standard procedure of detecting malaria parasites in suspicious specimens. Blood samples were taken from the enrolled participants. Thin and thick blood smears were prepared. After making the blood smears on slides, only thin smears were fixed with methanol. Thin and thick blood films were stained with the Giemsa method conveniently. The microscopic examination of stained slides was performed via Immersion oil at 1000× magnification (28). The microscopic evaluation of all participants was performed by two different groups (double-blind) to identify asymptomatic patients and individuals with low parasitemia.
3.3.2. Rapid Diagnostic Tests (RDTs)
The rapid malaria detection test is a diagnostic method used in association with the microscopic test to identify malaria, based on the formation of antigen/antibody complex. This prompt and accurate method relies on the immunochromatography mechanism.
All samples were tested with the First Response® Malaria Combo (pLDH/HRP2) card test (Premier Medical Corporation Ltd., Mumbai, India). According to the manufacturer’s instructions, five microliters of blood samples of the patient’s finger-prick were poured into the RDTs kit well. In the next step, three drops of buffer were added to the buffer well located on the kit to lyse RBCs. After 20 minutes, the results were analyzed according to lines that were visible in the test and control area (29).
3.3.3. Molecular Technique (Nested PCR)
As described by Snounou et al. (30), the molecular method of Nested PCR was implemented for the recognition of malaria parasites in samples based on the protocol.
The parasite DNA was extracted from specimens by the Genomic DNA Blood/Culture Cell Mini kit of “Yekta Tajhiz Azma” Iran Company. The Nested PCR technique used two sets of primers and was performed in two successive reactions, including genus identification and species identification of P. vivax and P. falciparum in taken samples of the study. In the first step of amplification (Nested PCR-1), 2 µL of extracted DNA of each sample was added to other reaction items. The reaction continued by a specific primer for Plasmodium (1200 bp). The reaction was done in the final volume of 50 µL, and the PCR was performed on the appropriate program by a thermocycler.
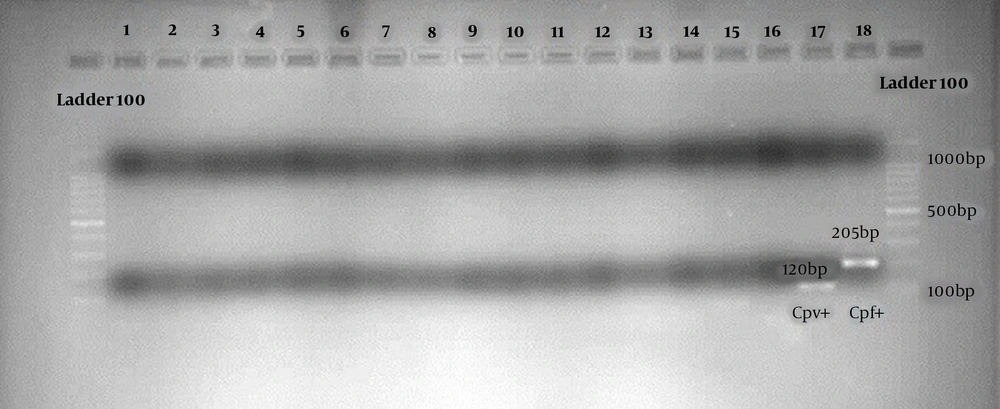
Nested PCR-2 involved the use of first-stage amplified product as a DNA template for the next reaction, and the species-specific primer was used to identify P. vivax (120 bp) and P. falciparum (205 bp). Each step was repeated for 25 - 30 cycles, and the annealing temperature was optimized at 72°C for both steps. Finally, to analyze the Nested PCR-2 products, electrophoresis was conducted in the presence of appropriate standard 100 bp molecular markers, and the gel photos were recorded by digital imaging for the final report (31). Positive and negative controls were used in each reaction series along with PCR running of the samples. The DNA extracted from the blood of healthy individuals who had not previously had malaria and had no history of traveling to endemic regions was applied as negative controls. For positive control preparation, the parasite DNA was extracted from blood samples of patients whose malaria had been confirmed by microscopic examination.
4. Results
Table 1 shows the demographic characteristics of the participants in this research. Of the 230 studied cases, 54.8% were females, and 454.2% were males. The age range was four to 65 years old, and the mean age was 24.5 years old. No positive cases of malaria parasites were found using microscopic, RDT, and molecular methods. It should be noted that the microscopic experiments were performed by double-blind microscopists (Table 2). In the molecular approach, positive and negative controls were used in each series of experiments (Figure 2). In summary, these results indicate that there were no asymptomatic malaria cases in Jask County despite using microscopic, serological, and molecular methods (Table 2).
| Status | Method | |||||
|---|---|---|---|---|---|---|
| Microscopic | RDT | Molecular | ||||
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Subject, No. (%) | 0 (0) | 230 (100) | 0 (0) | 230 (100) | 0 (0) | 230 (100) |
| Total | 230 (100) | |||||
5. Discussion
The elimination of malaria is one of the common goals of the Iranian health system and the World Health Organization. Active malaria case finding and detection of asymptomatic parasitic reservoirs are the most important strategies in the malaria elimination program (32). Despite using the molecular sensitive method for diagnosis, the results of this study showed that the asymptomatic parasitic reservoir was not observed in Jask. The absence of an asymptomatic parasitic reservoir indicates the existence of a robust surveillance system to diagnose and treat positive cases and monitor them.
To successfully apply the malaria elimination program, it is necessary to detect and monitor asymptomatic cases. Being a major challenge to the malaria elimination plan, asymptomatic malaria causes a reservoir, contributing to the ongoing local transmission of the disease. In this study, the PCR technique was used in addition to routine diagnostic methods (microscopic and RDT) because of the high sensitivity of the molecular method.
The findings of this study are in line with previous studies conducted in some parts of Iran, including Bashagard (15), Kerman (15, 16), Rudan (13), and Iranshahr (9). Despite using a sensitive molecular method for diagnosis, there have been no reports of asymptomatic cases in some of these cities. However, positive cases of asymptomatic malaria have been reported in cities of Minab (12), Bashagard (11), Iranshahr (8), and Bandar-Abbas (10). The most important reason to explain the disagreement between this study and previous studies may be the robust malaria surveillance system in the area (Jask).
Recently, a cross-sectional study was conducted on the situation of asymptomatic malaria among Iranian native and Afghan and Pakistani immigrants in the southeastern province of Sistan and Baluchistan. A total of 271 individuals were surveyed based on the microscopic method, Rapid Diagnostic test (RDT), and PCR techniques. Similar to our results, none of the examined samples was diagnosed as malaria-positive cases (33). Furthermore, there are similarities between the results reported in this study and those described by other researchers in Honduras (21), Sri Lanka (20), and India (19), which have not reported positive malaria cases despite the use of sensitive and robust molecular methods.
However, our findings are contrary to previous studies conducted in Bhutan (24), India (23), Thailand (18), China-Myanmar border (25), Ethiopia (22), and Brazil (17), which found asymptomatic malaria cases. A similar study was conducted in Ghana in 2018. Asymptomatic adult residents from five villages were screened for Plasmodium species using RDT and molecular techniques. Quite contrary to our results, the molecular prevalence of asymptomatic Plasmodium infection was 73%, and a 32% infection rate was detected by RDT (34). This discrepancy can be largely attributed to differences in the epidemiological characteristics and status of malaria transmission in the studied areas. Other possible explanations for this dissimilarity are differences in the genetic characteristics of the parasites and hosts, species diversity and vectorial capacity of vectors, and displacement or relocation of human populations (35).
The key strengths of this study are the simultaneous use of molecular sensitive techniques along with routine methods of malaria detection, as well as a wide field selected for sampling in the endemic area of Jask. The main limitations of this research include the small sample size and the impossibility to monitor the cases due to the cross-sectional design of the study. Despite the use of a molecular sensitive technique and routine methods of malaria detection, the asymptomatic parasitic reservoirs in the malaria-endemic area of Jask were not reported, indicating a successful implementation of the malaria elimination program in this area without worrying about low parasitemia and asymptomatic malaria cases.
According to the world malaria report 2020, local malaria transmission was not observed in Iran in 2018 and 2019. Due to the lack of local malaria transmission in 2020 and the completion of a three-year period for no local malaria transmission, conditions for obtaining a Malaria Eliminate Certificate have been provided by the WHO in Iran (4). Along with other similar research, the results of our study, as valid evidence, will facilitate the process of obtaining the certification of malaria-free for the country. In addition, the findings of this research contribute in many ways to identify the challenges of the malaria elimination program.
5.1 Conclusions
It can be concluded that Malaria Elimination Program is feasible in the Jask Region irrespective of asymptomatic parasitic reservoirs. The results also emphasize a robust and efficient malaria surveillance system to diagnose and treat the positive cases and monitor the treated cases successfully. Ongoing and continuous studies are recommended in the high-risk malarious area of Hormozgan province to monitor asymptomatic cases of malaria.