1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) should be considered a serious threat to global public health (1). The disease began in December 2019 in China, and more than 664,731 cases were confirmed worldwide, including 30,892 mortalities in 199 countries by March 29, 2020 (2). The disease has widely spread worldwide (3), and its mortality has been estimated at 2% - 3%; however, due to the unspecified number of infected patients, the actual rate of mortality is unclear (4).
Due to a large number of infected and dead cases, the development of approaches to control the epidemic condition, as well as effective and available drugs, should be considered as a priority for the health system and the governments (5). Nevertheless, despite the high mortality rate, there are no specific drugs to either treat or prevent the aggravation and serious complications of the disease. In this study, we presented three cases with COVID-19 admitted to the Emergency Department of Imam Reza Hospital, Mashhad, Iran.
2. Case Presentation
2.1. Case 1
An 18-year-old woman with a bad general condition and symptoms, including high-grade fever, chills, and respiratory symptoms, such as dry cough and shortness of breath, referred to the Imam Reza Hospital. She had no history of underlying diseases and drug administration. She had attended a wedding 2 days ago and denied any contact with people who had flu-like symptoms.
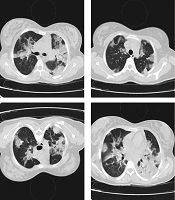
The patient was hospitalized in the intensive care unit (ICU) due to respiratory failure. On admission, she had a fever of 40°C, oxygen saturation of 85%, respiratory rate of 32 breaths/min, and a pulse rate of 120 bpm. The computed tomography (CT) scan of the chest was conducted, in which bilateral lung involvement and ground-glass opacity were observed (Figure 1). The laboratory findings are presented in Table 1.
| Laboratory Test | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| White blood cell, 1000/ML | 19.6 | 4.3 | 4.1 |
| Lymphocyte count, % | 4.5 | 20 | 10 |
| Haemoglobin, g/dL | 14.5 | 13.8 | 12.8 |
| Platelet count, cells/L | 340 | 374 | 126 |
| Lactic acid dehydrogenase, U/L | 890 | 875 | 548 |
| C-reactive protein, mg/L | 85 | 140 | 90 |
| Erythrocyte sedimentation rate, mm/h | 30 | 28 | 33 |
| Oxygen saturation, % | 85 | 81 | 88 |
| Heart rate | 120 | 110 | 100 |
| Respiratory rate | 32 | 30 | 32 |
| Fever, °C | 40 | 38.7 | 38 |
The case was one of the first patients suspected to COVID-19 in the outbreak, and polymerase chain reaction (PCR) diagnostic kits for the diagnosis COVID-19 were not available; therefore, the specimens of nasopharyngeal swab were tested for adenovirus, Mycoplasma pneumonia, Chlamydia pneumonia, human parainfluenza virus (HPIV), respiratory syncytial virus (RSV), influenza A (H1N1 and H3N2) virus, and influenza B virus. The results of all the aforementioned tests were negative. As a result, COVID-19 was considered a possible diagnosis due to lymphopenia, the pattern of chest CT scan, and patient history. The clinical status was monitored, and the patient received supportive care.
The treatment of COVID-19 was initiated by the administration of hydroxychloroquine (HCQ), Kaletra (lopinavir/ritonavir; 200/50 mg), oseltamivir, ceftriaxone, and azithromycin and continued for 4 days. Noninvasive ventilation (NIV) was performed due to the lack of response to the treatment and respiratory distress; however, respiratory distress was developed. Ceftriaxone was replaced with a broad-spectrum antibiotic due to persistent fever. Ribavirin and intravenous immunoglobulin (IVIG) (0.4 g/kg/day; 25 g/day) were added to the treatment regimen due to the development of respiratory distress. The IVIG was administered in five doses, respectively.
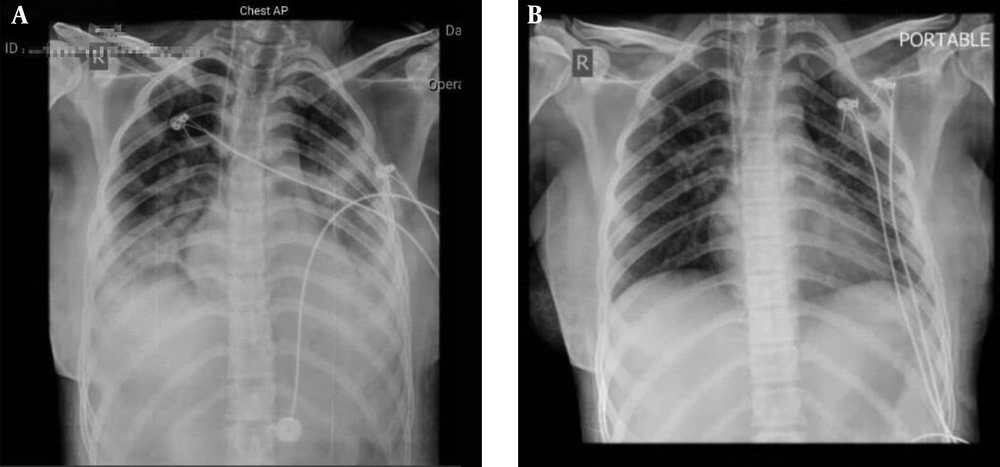
The patient’s fever was resolved 24 h after the administration of the treatment regimen. An increase in lymphocyte count and a decrease in lactate dehydrogenase and C-reactive protein were observed. Moreover, oxygen saturation increased to 92%. The chest X-ray showed remarkable recovery 14 days after the admission, and no adverse events were reported (Figure 2). The clinical status of the patient was improved, and supplemental oxygen was disconnected. Finally, the case was discharged with a good general condition.
2.2. Case 2
A 38-year-old man with respiratory distress referred to the Imam Reza Hospital. He had no history of underlying diseases and drug administration. Recently, he traveled to Iranshahr, Sistan and Baluchestan Province, Iran.
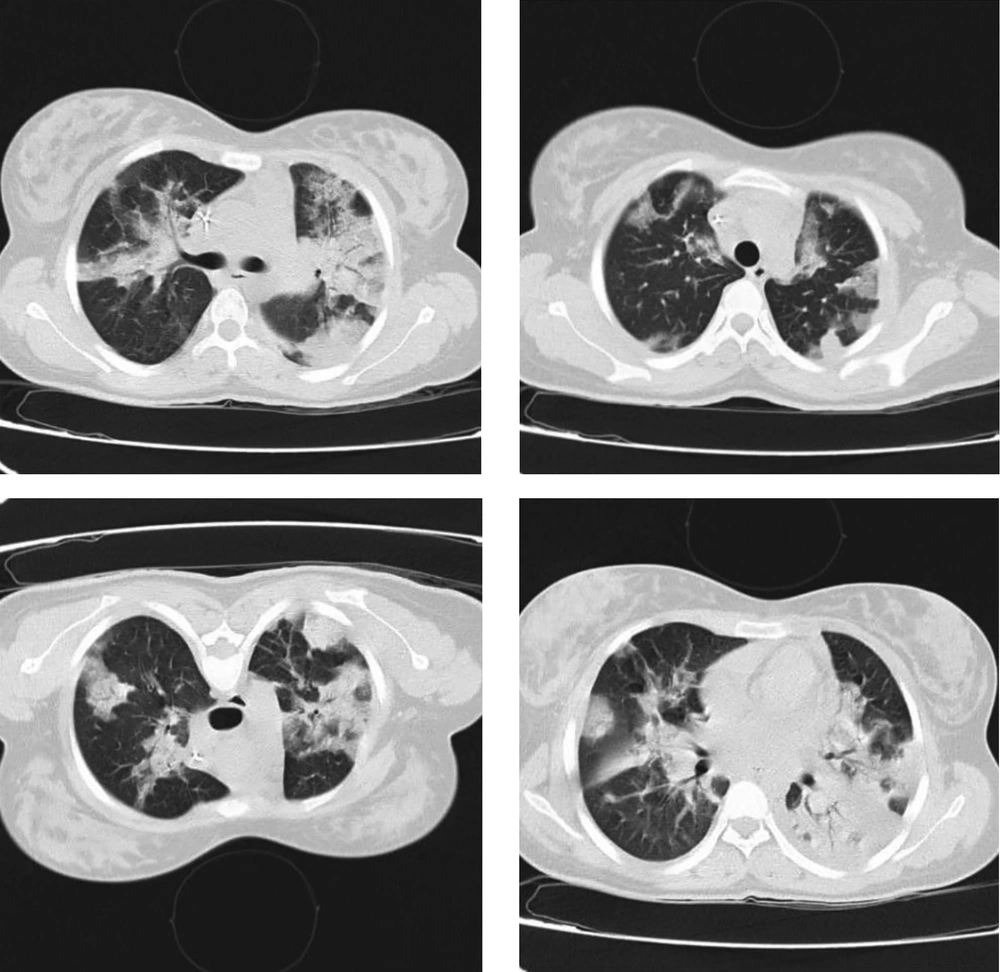
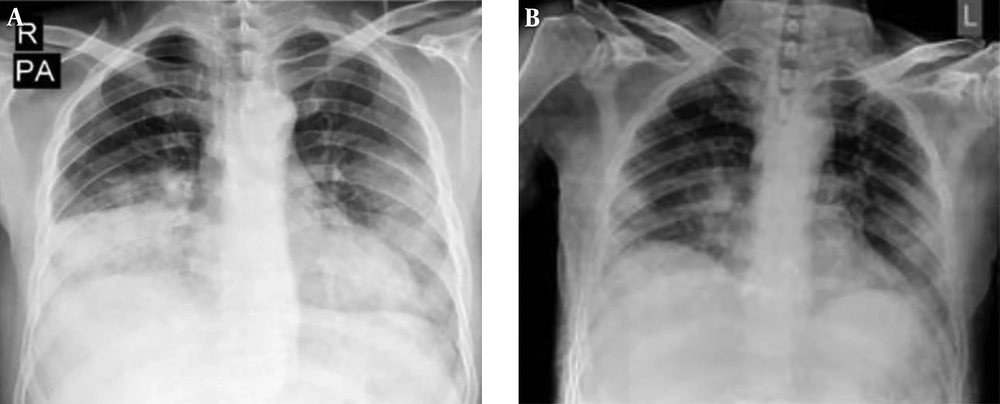
He reported fever and chills, weakness, dry cough, and shortness of breath started a week before the admission. Tachypnea was reported as 30 breaths/min. He had a fever of 38.7°C and an oxygen saturation of 81%. In addition, lung involvement was observed in the CT scan of the chest and chest X-ray (Figure 3).
The clinical status was monitored, and the patient received supportive care. The case was hospitalized in the ICU, and COVID-19 was considered as a possible diagnosis. Due to the lack of PCR diagnostic kits for the diagnosis of COVID-19, screening for multiple respiratory pathogens, including influenza A and B viruses, RSV, adenovirus, HPIV, M. pneumonia, and C. pneumonia, were performed. The results of all the aforementioned tests were negative. Therefore, COVID-19 was considered as a possible diagnosis.
He was treated with the administration of HCQ, Kaletra (200/50 mg), oseltamivir, ceftriaxone, and azithromycin for 3 days. The NIV was conducted due to no response to the treatment and increasing respiratory distress. As a result, ribavirin and IVIG (0.4 g/kg/day for five doses; 30 g/day) were added to the treatment regimen.
The patient fever was resolved 24 h after the initiation of the treatment regimen, and an increase in lymphopenia and oxygen saturation up to 93% was reported. Remarkable recovery after 14 days was observed in the chest X-ray, and the case was discharged with a good general condition (Figure 4).
2.3. Case 3
A 24-year-old man with symptoms, including high-grade fever, chills, dry cough, and shortness of breath, since 10 days ago referred to the Imam Reza Hospital. He had no history of underlying diseases and drug administration. However, he had recently traveled to Qom, Iran. He had a fever of 38°C and an oxygen saturation of 88% (Table 1). The patient was hospitalized in the emergency department. The CT scan of the chest was conducted, in which bilateral lung involvement was observed.
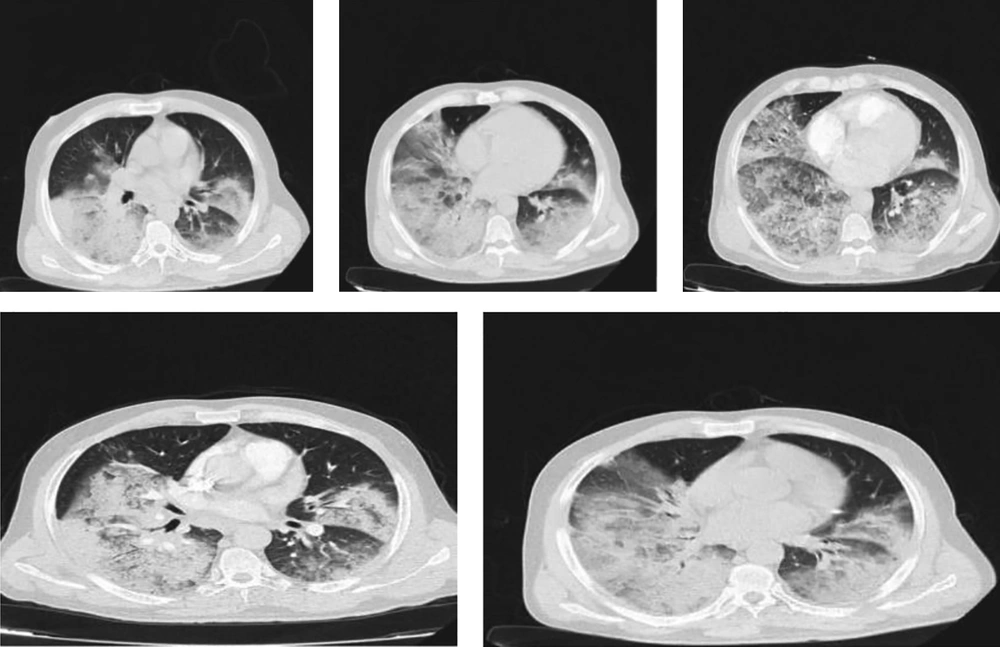
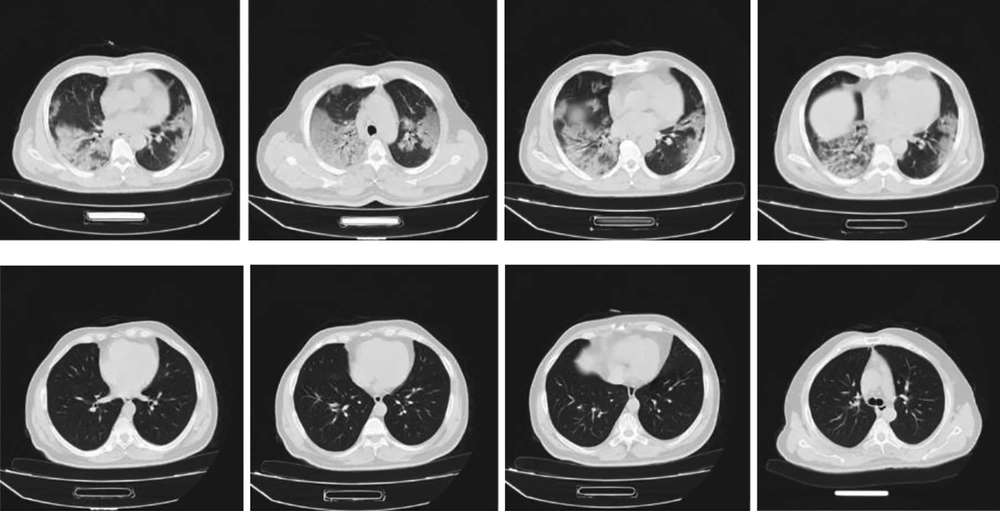
Due to lymphopenia, the pattern of chest CT scan, and patient history, COVID-19 was considered as a probable diagnosis, and the nasopharyngeal swab test for the COVID-19 nucleic acids was positive. He was treated with the administration of HCQ, Kaletra, oseltamivir, ceftriaxone, azithromycin, ribavirin, and early IVIG (0.4 g/kg/day, 30 g/day). The IVIG was administered in three doses, and the antiviral therapy was given for 9 days. The next CT scan of the chest was completely normal, an increase in lymphocyte count and a decrease in lactate dehydrogenase and C-reactive protein were observed. Moreover, oxygen saturation increased to 98%, and the patient was discharged with a good general condition. Figure 5 depicts the CT scan of this patient in the first and last days of the treatment.
2.4. Literature Review
In this review, the case reports and case series on patients with COVID-19 were assessed through searching the databases, such as PubMed, ScienceDirect, and Google Scholar. The research process was accomplished using the keywords, including “COVID-19” and “Coronaviruses”. All case reports or case series published in English up to March 27 2020 were included in the study.
2.5. Findings
During the literature search, 27 patients with COVID-19 were identified in 14 studies, and 3 cases were reported in the present study. In this regard, 80 cases (66%) were reported in China, and 6 cases (11%) were observed in Europe (France: 3, UK: 2, and Spain: 1). Out of 27 cases, one case (0.4%) was reported in Australia, and another case (0.4%) was observed in Korea. The reported cases were within the age range of 26-84 years. In general, 48% (n = 13) and 52% (n = 14) of the cases were female and male, respectively.
Fever, sore throat, dry cough, fatigue, chills, and muscle pain were the common primary complications of the patients. The majority of the patients had no history of underlying diseases. Moreover, hypothyroidism and hypertension were observed in some cases. All the cases were discharged after antiviral therapy. The range of the length of hospital stay was 10 - 34 days. Kaletra, oseltamivir, arbidol, moxifloxacin, ceftriaxone, HCQ, azithromycin, and IVIG were the most common drugs used for the treatment of COVID-19 (Table 2).
| Authors/References | Country | Date | Age, y | Gender | Primary complications | Underline diseases | Antiviral therapy | Administered drug | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Cheng et al. (6) | Taiwan | January 20 | 55 | Female | Sore throat, dry cough, fatigue, and low-grade subjective fever | Hypothyroidism | Antitussive agent, oxygen supplement, and ceftriaxone | Oral amoxicillin/clavulanate | Discharged after 28 days |
| Kim (7) | Korea | January 20 | 54 | Male | Chills and muscle pain | No | - | Kaletra | Discharged after 18 days |
| Wang et al. (8) | China | December | 34 | Female | Fever | Hypothyroidism | Recombinant human interferon α1b atomized inhalation, ganciclovir, tebipenem, moxifloxacin, and methylprednisolone | - | Discharged after 18 days |
| Tian et al. (9) | China | February | 84 | Female | Admitted for the treatment evaluation of a tumor (1.5 cm) in the right middle lobe of the lung | Hypertension for 30 years, type II diabetes, lung cancer | Antibiotics and assisted oxygenation | - | Discharged |
| 73 | Male | Fever, drycough, chest tightness, and muscle pain | Hypertension for 20years and lung cancer | - | - | Discharged after 20 days | |||
| Bernard Stoecklin et al. (10) | France | January 10 - 24 | 48 | Male | Fever, headache, and cough | No | - | - | Discharged |
| 31 | Male | Fever, chills, fatigue, conjunctivitis | No | - | - | Discharged | |||
| 30 | Female | Fever, chills, fatigue, and cough | No | - | - | Discharged | |||
| Chen et al. (11) | China | January17 | 46 | Female | Fever | No | Oral antibiotics | Oseltamivir, arbidol, lopinavir/ritonavir, and moxifloxacin | Discharged after 22 days |
| Qin et al. (12) | China | January 13 - 20 | 57 | Male | Fever and sore throat | No | Antiviral, anti-inflammatory, and symptomatic treatments for 2 weeks | - | Discharged |
| 56 | Male | Fever, fatigue, and dizziness | Surgery for lung repair and rib fracture internal fixation | Antiviral and anti-inflammatory drugs | - | Discharged | |||
| 61 | Female | Back pain and dry cough | No | Antiviral and anti-inflammatory drugs | - | Discharged | |||
| 48 | Female | Fever, chills, dry cough, myalgia, and fatigue | No | Antiviral and anti-infective treatments | - | Discharged | |||
| Thevarajan et al. (13) | Australia | 48 | Female | Lethargy, sore throat, dry cough, pleuritic chest pain, mild dyspnea,and subjective fever | No | - | - | Discharged | |
| Li et al. (14) | China | January 23 | 51 | Male | Intermittent fever, chills, fatigue, poor appetite, and diarrhea | Heart transplant | IVIG1, methylprednisolone, an immunosuppressive drug, moxifloxacin, and arbidol | - | Discharged after 34 days |
| January 25 | 48 | Male | Fever | Heart transplant | Ceftriaxone sodium, oral moxifloxacin, and tacrolimus | - | Discharged after 11 days | ||
| Lillie et al. (15) | UK | January 23 | 50 | Female | Fever and malaise, sore throat, and dry cough | No | Antibiotic and antiviral therapies | - | Discharged |
| January 28 | 26 | Male | Fever, myalgia, malaise, and sinus congestion | No | Antibiotic therapy (co-amoxiclav500/125 mg) and antiviral therapy | - | Discharged | ||
| Guillen et al. (16) | Spain | February 28 | 50 | Male | Fever and vomiting | Renal disease due to immunoglobulin A nephropathy and elective splenectomy | Lopinavir/ritonavir, calcineurin inhibitors, ceftaroline, and meropenem | Lopinavir/ritonavir, interferon beta, and HCQ | Discharged |
| Ni et al. (4) | China | January 17 | 51 | Female | General malaise and coldness | No | Intravenous injection of cefotaxime, oseltamivir, moxifloxacinarbidol, and granules | IVIG (5 g per day) and dexamethasone (5 mg once to twice a day) | Discharged after 14 days |
| January 21 | 27 | Female | Mild weakness, diarrhea, and low-grade fever | Loxoprofen | Shuang-Huang-Lianoral liquid | Discharged after 14 days | |||
| January 26 | 53 | Male | Mild diarrhea, vomiting,and fever | No | Shuang-Huang-Lian oral liquid, moxifloxacin, and arbidol | - | Discharged after 11 days | ||
| Zhu et al. (17) | China | January 27 | 44 | Male | Cough, sore throat, headache,fatigue, muscle ache, and joint ache | No | - | - | Discharged after 17 days |
| Wu et al. (18) | China | February | 54 | Female | Fever | No | Oseltamivir | - | Discharged |
| Cao et al. (19) | China | January22 | 56 | Male | Fever for 2days before admission | No | Oseltamivir andazithromycin | IVIG | Discharged after 13 days |
| January 28 | 34 | Male | Fever and dry cough for 10 days | Hypertension | - | IVIG | Discharged after 7 days | ||
| January 24 | 35 | Female | Low-grade fever and malaise | No | Kaletra | IVIG | Discharge after 15 days | ||
| Our cases | Iran | February | 18 | Female | Severe fever and chills, and respiratory symptoms, such as dry cough and shortness of breath | No | HCQ, Kaletra, oseltamivir, Ceftriaxone, and azithromycin | Ribavirin and IVIG | Discharged after 14 days |
| 38 | Male | Fever and chills, fatigue and malaise, dry cough, and shortness of breath | No | HCQ, Kaletra, oseltamivir, ceftriaxone, and azithromycin | Ribavirin and IVIG | Discharged after 14 days | |||
| 24 | Male | Severe fever and chills, dry cough, and shortness of breath since 10 days ago | No | HCQ, Kaletra, oseltamivir, ceftriaxone, and azithromycin | Ribavirin and IVIG | Discharged after 9 days |
Abbreviations: HCQ, hydroxychloroquine; IVIG, intravenous immunoglobulin.
3. Discussion
The COVID-19 is a highly infectious disease with a relatively high rate of mortality and prevalence rate. To date, a wide clinical spectrum of COVID-19 infection from asymptomatic to pulmonary infiltrations has been introduced. Although many individuals have been infected with COVID-19 around the world over the past 3 months, clinical information and pathologic changes in these patients remain unknown.
In addition, there is limited information on the manifestations of this disease. The nature of COVID-19 infection does not resemble other previously known coronaviruses. Due to the lack of an exact definition of the disease, its clinical management is very difficult (20, 21). In this study, we reported three cases with COVID-19 who were successfully treated with a high dose of IVIG.
The development track of COVID-19 is similar among symptomatic patients. Mild or moderate symptoms, including fever and chills, sore throat, fatigue and malaise, dry cough, and shortness of breath, are the most common primary symptoms of COVID-19, as observed in the reported cases of the current study. The primary symptoms are similar to those reported for the common cold, which usually last for 3 to 7 days (20, 22). When the disease becomes quite prominent, respiratory distress, developed fever, and in some cases, gastrointestinal symptoms appear (23).
Based on the results of the present study and other observations, when acute respiratory distress syndrome is developed, most of the lesions are started from the peripheral of the lung, especially in the subpleural areas, which suggested hematogenous or lymphatic distribution or development of pathogenic factors (18). Starting phase, spanning the acquisitions of the virus, and subsequent viremia can be considered as the three main phases of COVID-19. Moreover, the accelerating phase is observed in some patients, in which secondary damage to organs, such as the lung, heart, and gastrointestinal tract is observed. Accordingly, the management strategies for the patients should be regarded in terms of the disease phase (24).
Several treatments are suggested for COVID-19. Recently, remdesivir (GS-5734) and chloroquine (CQ) phosphate are suggested as effective substances for the inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vitro (5, 25). Based on a report, the clinical condition of a patient infected by SARS-CoV-2 in the USA improved after taking remdesivir (26), and a phase III clinical trial was carried out in Wuhan, China, for the assessment of the drug against SARS-CoV-2. However, it is not available for the treatment of a very large number of people infected with COVID-19.
The CQ (N4-[7-Chloro-4-quinolinyl]-N1,N1-diethyl-1,4-pentanediamine) is suggested as a choice of treatment for a large number of patients due to its availability, safety, and low cost. Based on the guidelines for the diagnosis and treatment of COVID-19, it has been added to the list of trial drugs against COVID-19 (27). The drug has been used to treat malaria and amebiasis. However, the overdose of CQ may lead to acute poisoning and mortality. Moreover, the use of CQ in clinical practice greatly decreased in the past years leading to the reduction of its production (28).
A much less toxic derivative of CQ is HCQ, which has been widely used recently. It is extensively available for the treatment of autoimmune diseases, such as rheumatoid arthritis. The HCQ is suggested as a potent candidate for the treatment of the infection by SARS-CoV-2 due to similar chemical structures and acting mechanisms of CQ and HCQ. During the outbreak of COVID-19, HCQ was used to treat COVID-19, as it was reported in seven clinical trials registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn) over February 23, 2020.
The IVIG is a safe and successful anti-inflammatory agent with an antiviral activity that has been extensively used in the treatment of autoimmune diseases. The production of cytokines, especially pro-inflammatory factors, decreased due to the use of IVIG. The agent plays a role in attenuating the inflammatory response in patients with COVID-19 (29).
In this study, a high dose of IVIG was used as a potent and safe treatment for three patients with COVID-19. A well-established practice in immune modulation therapy for other diseases was considered for the determination of the dosage of IVIG (30). In this regard, the right time to choose antiviral therapy is critical, which should be selected based on the phase of the disease. The first few days of deterioration are the critical point when the potent suppression of inflammatory cascade occurs, which can save the patients from fatal immune-mediated injuries. The progression of the COVID-19 cascade can be blocked by the use of IVIG when it is administered at an appropriate point (18).
The potential cardiovascular or renal diseases of patients with COVID-19 were considered in the determination of an IVIG dose. All reported cases were improved and their temperature decreased. Furthermore, breathing difficulties were resolved following the administration of IVIG during 3 - 6 days, and no adverse event was reported by the cases of the present study after the treatment.
However, other antiviral drugs were used in the first phase of treatment for two cases without using ribavirin and IVIG. In both cases, the improvement process was not observed before adding IVIG. However, three cases who received IVIG since the first stage of treatment were reported with a shorter course of treatment. The treatment regimens were similar among the cases of the current study; therefore, it is suggested to use the treatment regimens for other patients with COVID-19.
One similar study carried out by Cao et al. reported three cases with COVID-19 treated with IVIG (0.4 - 0.5 g/kg daily). All cases improved and reported no adverse event. The fever was resolved 1 to 2 days after the use of IVIG, and breathing problems were recovered within 3 - 5 days after treatment (19). Moreover, IVIG was utilized in another study by Ni et al. (4) for the treatment of a woman with COVID-19. They showed the effectiveness of IVIG (5 g/kg/day). In the aforementioned study, Shuang-Huang-Lian oral liquid was administered to the case, who did not respond to IVIG, dexamethasone, and antivirus agents.
The IVIG is polyclonal immunoglobulin G derived from the large pools of normal donor serum, containing a great number of bioactive moieties. The mechanism of action of this drug has not yet been completely identified. The immunomodulatory mechanism of IVIG, including Fc-mediated and Fab-mediated approaches, is suggested in several theories (31, 32). In this regard, the time of using IVIG is very important; accordingly, when it is administered in the first stage of the disease, the best outcomes are observed. However, it may have no benefit when is used in patients with developed systemic damage.
Although positive results were observed after the use of IVIG in patients with COVID-19, the present study was limited to three cases, and there are very few studies assessing the effectiveness of this agent in patients with COVID-19. The efficacy of using IVIG in patients with COVID-19 and the Middle East respiratory syndrome is shown in previous studies. It can be considered as a treatment of choice for patients with COVID-19 who are at the early stage of the disease due to the safety profile of the agent and its effect on the improvement of passive immunity and modulation of immune inflammation.
Beneficial effects were observed regarding IVIG, especially in cases, for whom the drug was administered at the initiation of the treatment regimen. It was also effective in COVID-19 patients with rapid disease spreading. A quazi experimental study (thesis ID: IR.MUMS.REC.1399.013) will be carried out between March and April 2020 to collect further evidence on the effectiveness of IVIG in the control and treatment of patients with COVID-19.
3.1. Conclusions
In summary, the obtained results indicated that IVIG can efficiently inhibit COVID-19 infection. The drug was effective against the disease; therefore, it is recommended to conduct clinical trials to assess the effectiveness of IVIG.