1. Introduction
Despite its worldwide report, the neurological manifestation of COVID-19 has not been well defined. From 221 COVID-19 cases in a single center of China, Li et al. (1) reported 11 ischemic stroke, 1 cerebral venous thrombosis, and 1 hemorrhage stroke. Mao et al. (2) reported neurological symptoms in 78 (36.4%) of 214 Chinese patients, including dizziness, headache, hypogeusia, hyposmia, ataxia, seizure, ischemic stroke, and 1 case of cerebral hemorrhage. Also, Helms et al. (3) reported neurological signs (e.g., agitation, confusion, executive dysfunction, encephalic, allopathy, and ischemic stroke) in 49 of 58 French patients with severe COVID-19. Several studies reported encephalitis, meningitis, seizure, cerebral vein thrombosis, and Guillan-Barre syndrome associated with COVID-19 infection (4-8). Sharifi-Razavi et al. (9) reported three cases of adult patients with ischemic stroke and novel coronavirus 2019 infection.
In this study, we first reported the association of cerebral hemorrhage with COVID-19 at Bou-Ali Sina Hospital on March 15, 2020 (10). Recently, other studies have reported the association of cerebral hemorrhage and COVID-19 (11, 12). Second, we described the clinical symptoms and also radiological and laboratorial characteristics of patients hospitalized to Bou-Ali Sina Hospital with ICH in association with COVID-19.
2. Case Presentation
2.1. Case 1
On March 28, 2020, a 78-year-old woman was taken to the emergency room with acute left hemiparesis, nausea, and impaired orientation. She had a low-grade fever but no respiratory or gastrointestinal symptoms. She had a history of ischemic heart disease (IHD) and was under treatment with aspirin and atorvastatin. There was no prior history of trauma. On the admission time, the patient was confused, and the first vital signs were blood pressure (BP): 130/76, heart rate (HR):62, respiratory rate (RR) :14 breath/min, body temperature (T) 38.3 C, and O2 saturation 97% on room air.
Neurological examination revealed right hemiparesis: Medical Research Council (MRC) scale grade 3. Deep tendon reflexes (DTR) were 2+ brisk at left, and 1+ and cutaneous plantar responses were extensor on the left side. There was no sign consistent with the hemorrhagic process on the whole body examination. Cardiopulmonary and abdominal examinations were normal as well.
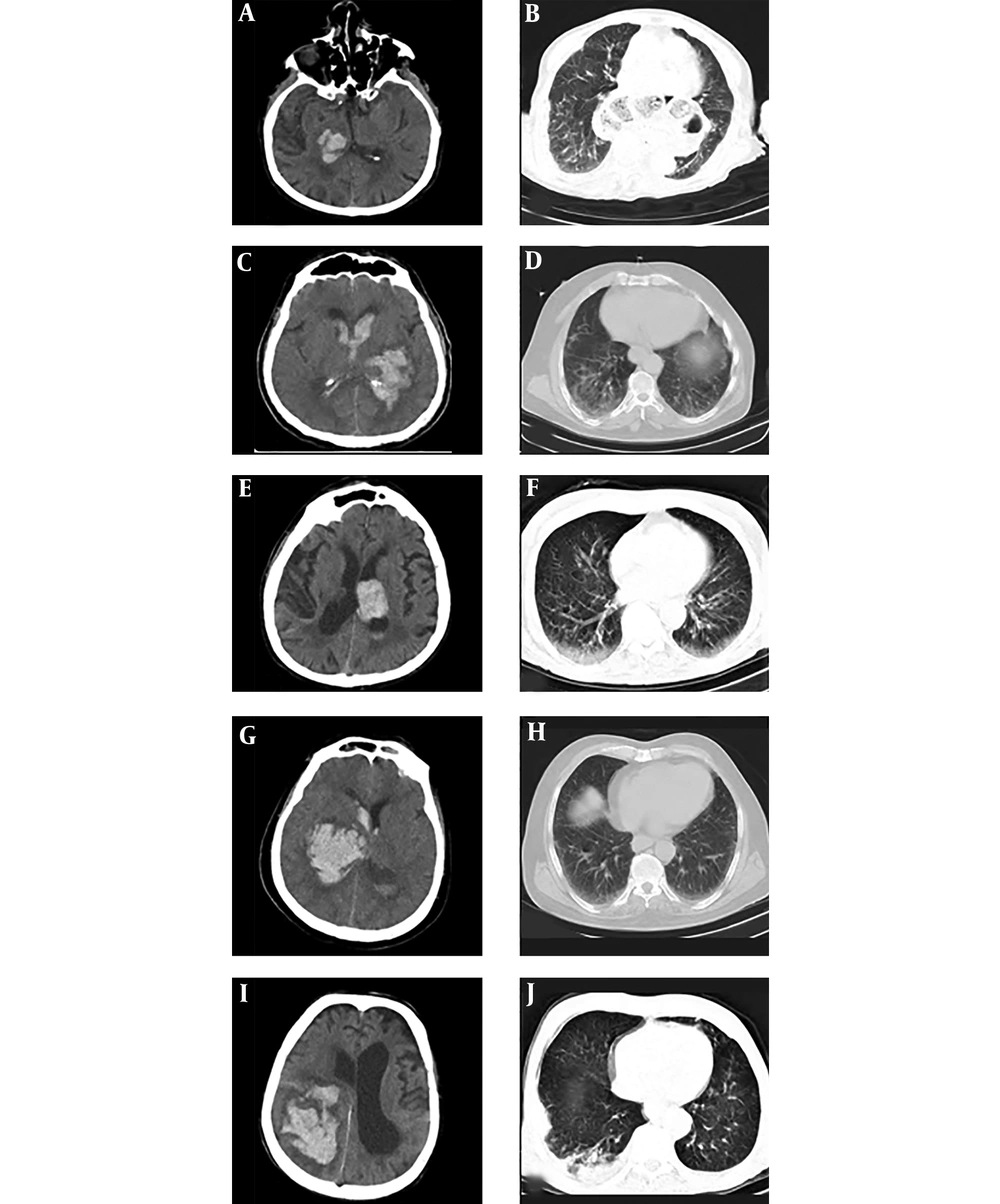
The laboratory test findings were as follows: white blood cell (WBC): 5000/ml (neutrophil:2600, lymphocyte:1650), platelet: 250000/microL, C-reactive protein (CRP):3 mg/dL, erythrocyte sedimentation rate (ESR) 21 mm/h, lactate dehydrogenase (LDH): 345 IU/L, partial thromboplastin time (PTT): 32 sec, prothrombin time (PT):14.6 sec, and INR: 1.2. Other routine laboratory tests (e.g., electrocardiogram (ECG) and echocardiography) were normal. Real-time polymerase chain reaction (RT-PCR) from a nasopharyngeal swab sample was COVID-19 positive. A non-contrast Brain CT scan demonstrated medium size hemorrhage in the right thalamus (Figure 1A). Lung computed tomography (CT) scan revealed mild ground-glass opacity and subpleural involvement in the right lungs (Figure 1B). The patient was taken to the intensive care unit (ICU) and treated with lopinavir/ritonavir (LPV/RTV) and conservative management for Intra Cerebral Hemorrhage (ICH).
2.2. Case 2
On March 31, 2020, a 62-year-old man with a history of generalized weakness for 3 days was presented with acute right hemiplegia and profound loss of consciousness. Past medical history revealed hypertension (HTN), and diabetes mellitus (DM) treated with losartan, glibenclamide, and hydrochlorthiazide. At the admission, BP was 165/95, HR 108, RR 22 breaths/min, body T 38.1°C, and O2 saturation 82 % on room air. After the primary care supports, he was immediately transferred to ICU, intubated for mechanical ventilation. Neurological examination showed the right hemiplegia (MRC scale grade 0) and bilateral plantar reflex extensor response. There was no skin rashes, mucosal hemorrhages, or any signs of trauma after examining of whole body.
First laboratory test showed WBC: 9720/mL (neutrophil: 48609 and lymphocyte: 3420), platelet: 316000 micro/L, CRP:5 mg/dl, ESR: 18mm, PTT: 30 sec, PT: 14.7 sec, INR: 1.3, and LDH: 387 IU/L. There was no acute change in ECG. He was not tested for COVID-19. Non-contrast brain CT scan demonstrated large left basal ganglia hemorrhage accompanied by intraventricular hemorrhage (Figure 1C). Lung CT showed different bilateral ground-glass opacities that are typical for COVID-19 infection (Figure 1D). This patient was treated with meropenem, Vancomycin, hydroxychloroquine, and conservative management for ICH.
2.3. Case 3
On April 8, 2020, a 78-year-old man referred to the emergency room with acute right hemiplegia, nausea, vomiting, and confusion. He had dry coughs in the past few days. He had a history of ischemic stroke, HTN, and dyslipidemia. He was taking aspirin, atorvastatin, losartan, metoprolol, and folic acid supplement. He had no prior history of trauma. On the admission time, the patient had confusion, and the first vital sign showed the following: BP: 170/90, HR: 52, RR: 15 breaths/min, T 37.8°C, and O2 saturation 97% on room air. Neurological examination revealed that spastic hemiplegia could affect the left side of the body due to previous ischemic stroke (MRC scale grade 1) at both upper and lower limbs and acute right flaccid hemiparesis (MRC scale grade 3). Sensory and cerebellar examinations were impossible to evaluate due to the decreased level of consciousness. DTRs were brisk at left and 1+ at right upper and lower limbs. Cutaneous plantar responses were extensor on both sides. There were no skin rashes, mucosal hemorrhages, or any signs of trauma after examining of whole body. The first laboratory test showed WBC: 9900 (neutrophil 7425, lymphocyte 1980). Platelet: 151000/microL, CRP: 18 mg/dL, ESR: 42 mm/h, PTT: 30 sec, PT: 12.5 sec, and INR: 1. Also, other routine lab tests, such as electrocardiogram and echocardiography, were normal. RT-PCR from a nasopharyngeal swab sample was positive for COVID-19. Non-contrast brain CT scan demonstrated a large thalamic hemorrhage on the left side with intraventricular extension (Figure 1E). A Lung CT scan showed diffuse ground-glass opacities in the basal of both lungs and more prominent on the right side (The The patient was admitted to the intensive care unit because of an altered level of consciousness and respiratory failure which needed to get oxygen through the CPAP.
This patient was treated with hydroxyl chloroquine, lopinavir/ritonavir (LPV/RTV), azithromycin, and conservative management for ICH.
2.4. Case 4
On April 9, 2020, a 67-year-old woman was presented with acute left hemiplegia, global aphasia, nausea, vomiting, and rapidly progressive loss of consciousness. She had a history of IHD, HTN, and DM. She was taking aspirin 80 mg, clopidogrel 75 mg, losartan 50 mg BID, and metformin 500 TID. She had no prior history of trauma. On the admission day, the patient was confused with BP, 240/120, HR, 86, RR, 16 breath/min, body T, 36.8°C, and O2 saturation 93% on room air. Neurological examination showed anisocoric pupils with a smaller left pupil and left hemiplegia (MRC scale grade 1). Sensory and cerebellar examinations could not be evaluated due to her decreased level of consciousness. The DTR of her upper and lower extremities was 2+. Cutaneous plantar responses were extensor on both sides. On cardiopulmonary examination, the patient had shortness of breath. There were no skin rashes, skin and mucosal hemorrhages, or any signs of trauma after examining the whole body.
The laboratory test findings were follows: serum glucose, 217 mg/dL, marked lymphopenia (WBC: 13800, neutrophil: 12420, lymphocyte: 828), platelet, 208000/microfiber, CRP: 4 mg/dL, PTT: 30 sec, PT: 12.5 sec, INR: 1. The electrocardiogram and electrocardiography showed moderate Left ventricular hypertrophy. RT-PCR from a nasopharyngeal swab sample was positive for COVID-19 infection. Non-contrast brain CT scan demonstrated Large right thalamus hemorrhage and intraventricular hemorrhage (A A Lung CT scan revealed mild ground-glass opacities in the basal of both lungs (Figure 1H).
The patient was admitted to the ICU and intubated due to a decreased level of Oxygen saturation and progressive loss of consciousness. She was treated with hydroxyl chloroquine, beta interferon, azithromycin, ceftriaxone, clindamycin, montelukast, and conservative management for ICH.
2.5. Case 5
On April 10, 2020, an 85-year-old man with a history of fever from 5 days ago, no evaluation and treatment, was referred to the emergency room with acute left hemiplegia, dysarthria, and impaired orientation. He did not have any history of anticoagulant our antiplatelet consumption not did he have any prior history of trauma, hypertension, and other medical disorders. On the admission time, the patient was confused, and the first vital sign discovered the following: BP: 120/70, HR: 98, RR: 26 breath/min, body T. 37.1°C, and O2 saturation 80% on room air. At the time of hospitalization, the patient was confused, and the neurological examination showed left hemiplegia (MRC scale grade 2). Sensory and cerebellar examinations could not be conducted. DTR was 1+ at left limbs and 2+ at right limbs. The cutaneous plantar response was extensor on the left side. No signs of trauma and hemorrhage were detected after the whole-body examination.
Primary laboratory test results indicated Leukocytosis (WBC: 14300, neutrophil: 12155; lymphocyte: 2145). Red blood cell: 2.93 × 106/micro liter; hemoglobin: 8.2 g/dL; platelate: 150000/microL; CRP: 23 mg/dL; ESR: 45 mm/h; creatinine: 2.1 mg/dL; PTT: 30 sec; PT: 15 sec; INR: 1.2; lactate dehydrogenase (LDH): 717 IU/L. Electrocardiogram and electrocardiogram tests were normal. RT-PCR from a nasopharyngeal swab sample was positive. A non-contrast Brain CT scan demonstrated an intraparenchymal hemorrhage in the right parieto-occipital lob with intraventricular extension. (Figure 1I). Spiral lung CT scan revealed ground-glass opacities in the basal of both lungs superimposed with consolidation in the right side (Figure 1J).
The patient was transferred to the intensive care unit and intubated due to his decreased level of Oxygen saturation and difficulty in breathing. He was treated with hydroxyl chloroquine, lopinavir/ritonavir (LPV/RTV), Azithromycin, ceftriaxone, clindamycin, and conservative management for ICH.
3. Discussion
In this study, we reported five infected patients with COVID-19 associated with intracerebral hemorrhage. There was no history of coagulopathy, anticoagulant consumption, and trauma in all cases.
Despite having no history of HTN, case 1 consumed antiplatelet. She had mild lung involvement. There was no reasonable relation between ICH and COVID-19. She did not need aggressive O2 therapy and was discharged with no important complications after 10 days.
Case 2 had a history of HTN, but he did not take antiplatelet. He needed early intubation and mechanical ventilation. After about 12 hours, he suffered massive upper gastrointestinal hemorrhage, reduced platelet count, and expired 20 hours after hospitalization. His lung involvement was not severe; CRP was negative, and lymphocyte count was normal; there was an underline medical condition, while ICH size was large with intraventricular hemorrhage. On the other hand, we had no microbiological confirmation for COVID-19, and only the radiological aspect was in favor of this infection.
Therefore, it seems his serious condition and final outcome happened due to cerebral hemorrhage, but not for COVID-19 infection.
Case 3 had a history of HTN, antiplatelet consumption, large ICH size, and moderate lung involvement. He first took O2 with CPAP, but he was intubated after 48 hours. During the hospitalization, he suffered ventilator-induced pneumonia, but gradually his condition improved, and after three weeks, the patient was discharged to home with a relatively stable condition.
Case 4 had a history of HTN and double antiplatelet therapy. She had marked lymphopenia, but her lung involvement was mild. She was admitted to ICU, and after a few hours, she was intubated due to brain edema and midline shift.
Case 5 had no risk factors for hemorrhage but advanced age. His ICH size was large, and lung involvement was severe, with suspicion of bacterial superinfection. It was a history of 5-day fever before hospitalization. The patient was under mechanical ventilation and, eventually, poor prognosis. He was passed away. We could not find any association between patients’ lab data, imaging findings, and patient prognosis for COVID-19 infection.
It was demonstrated that coronaviruses, and especially β-coronaviruses, to which the COVID-19 belongs do not limit their presence to the respiratory tract and frequently invade the CNS (13, 14). Literature shows that Angiotensin-converting enzyme 2 (ACE2) receptors, but not the exclusive, is a site of entry of the virus into the cell. ACE2 is expressed in many organs such as the brain. Brain ACE system involvement and dysfunction potentially can lead to autoregulation disruption and high blood pressure spikes and resulting in rupture of the vessel wall (13).
Another aspect of COVID-19 infection is its effect as a systemic inflammatory storm with a massive release of cytokines, chemokines, and other inflammation signals with a subsequent significant break of blood-brain barrier (BBB) and promoting neuroinflammation (15). Although the neuroinflammation and BBB disruption are essential in ischemic stroke and neurodegenerative disorders (16, 17), their impression on ICH is unknown. Vasospasm, due to the release of inflammatory cytokine and coagulopathy disorder, especially in severely ill patients, is a speculated mechanism of the cerebrovascular attacks.
More studies and autopsy evaluations are necessary for achieving an accurate proof of the causative relationship between COVID-19 and ICH.
3.1. Conclusions
Although neurological manifestations of COVID-19 have not been well defined, it is possible that a number of these patients, particularly those who suffer from a severe illness, have central nervous system involvement. Thus, neurologists should be aware of the likelihood of any neurological symptoms of COVID-19 infection.