1. Background
Novel Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is an emerging infectious disease that first manifested in December 2019 in Wuhan and subsequently spread worldwide. Coronavirus disease 2019 has become a major public health problem resulting in considerable morbidity and mortality. As of April 30, 2020, there were 3,090,445 confirmed cases and 217,769 deaths in the world (1).
In addition to the severe state of pneumonia, the new coronavirus can cause multiple organ failure by attacking several important organs. The heart is one of these organs. It is very likely that viral myocarditis and myocardial damage are involved and may even be one of the main causes of death from COVID-19. Regardless of previous cardiac history, heart failure is described in severe cases of COVID-19 (2, 3). This increases the levels of myocardial markers, especially cardiac N-terminal pro-brain natriuretic peptide (NT-proBNP), and troponin I (cTnI), specifically in severe cases (4).
2. Objectives
In the present study, we tried to determine the role of laboratory indicators of heart injury including cardiac troponin I (cTnI), NT‐proBNP, and Lactate Dehydrogenase (LDH) in predicting the disease severity and outcome in 73 patients with COVID-19 at Cheikh Khalifa International University Hospital, Mohammed VI University of Health Sciences (UM6SS), Morocco.
3. Methods
In a retrospective study, we enrolled 149 patients from March 21, 2020, to April 24, 2020, at Cheikh Khalifa International University Hospital, Mohammed VI University of Health Sciences (UM6SS), Morocco. The final follow-up date was April 30, 2020. The laboratory confirmation of COVID-19 was done as recommended (5). We excluded 76 patients because of incomplete data, leaving 73 patients for final analysis. The Ethics Committees of the Mohammed VI University of Health Sciences approved this study.
The COVID-19 patients included in this study were diagnosed following the World Health Organization's interim guidelines (1). They were classified into three groups: Mild (39 cases), severe (17 cases), and critical (17 cases). The diagnostic criteria for mild cases were a positive result in the real-time fluorescence polymerase chain reaction of COVID-19 virus RNA nucleic acid test, fever, or other respiratory symptoms, besides imaging characteristics typical of lung involvement as an additional criterion (6). For severe cases, at least one of the following criteria must be met: (a) Respiratory depression (breathing rate > 30 breaths/min), (b) need for oxygen treatment or mechanical ventilation, and (c) SpO2 < 90 % of ambient air (1). Critical cases must meet at least one of the following additional criteria: (a) Respiratory failure requiring mechanical ventilation, (b) shock, and (c) multiple organ failure (7). Confirmed COVID-19 cases were hospitalized and placed in isolation for treatment. We collected data on the first detection of laboratory parameters of cardiac lesions in these 73 patients with COVID-19 on admission. The cases without cardiac biomarkers, including cardiac troponin I (cTnI), NT-proBNP, and LDH, were excluded.
We used SPSS for statistical analysis. Differences between the groups were analyzed. The Pearson correlation coefficient was used the linear correlation analyses. The categorical variables were compared using the χ2 test. P value < 0.05 was considered statistically significant.
4. Results
4.1. Demographic and Clinical Characteristic
Data were collected from consecutive hospitalized COVID-19 patients. We excluded 76 patients because of incomplete data, leaving 58 discharged individuals and 15 dead ones for final analysis.
The mean age was 54.58 (17.62) years. The difference in sex and age between the three groups was significant (Table 1). Of the 73 patients, 58 (51.8%) had one or more cardiovascular risk factors. Hypertension (23 [33.8%]), diabetes (12 [17.6%]), and smoking (4 [5.9%]) were the most common cardiovascular risk factors and six patients had underlying Coronary Heart Disease (CHD) (Table 2). The most common heart-related symptoms were cough (42 [57.5%]), fever (39 [53.4%]), chest pain/tightness (34 [46.6%]), and shortness of breath (21 [28.8%]).
| Total Cases | Mild Group, (N = 39) | Severe Group, (N = 17) | Critical Group, (N = 17) | P Value | |
|---|---|---|---|---|---|
| Age (years) | 54.58 ± 17.62 | 49.59 ± 18.81 | 56.65 ± 16.85 | 63.94 ± 10.73 | 0.015 |
| Gender, % | 0.004 | ||||
| Men | 38.40 | 48.90 | 15.60 | 35.60 | |
| Women | 61.60 | 60.70 | 35.70 | 3.60 |
Coronavirus Disease 2019 Patients Based on Age and Gender
| Mild Group, (N = 39) | Severe Group, (N= 17) | Critical Group, (N = 17) | P Value | |
|---|---|---|---|---|
| Hypertension | 10 (43.5) | 5 (21.7) | 8 (34.8) | 0.186 |
| Diabetes | 5 (41.7) | 3 (25.0) | 4 (33.3) | 0.525 |
| Smoking | 1 (25.0) | 2 (50.0) | 1 (25.0) | 0.376 |
| Coronaropathy | 1 (16.7) | 1 (16.7) | 4 (66.7) | 0.02 |
Cardiovascular Risk Factors and Cardiovascular Disease of Coronavirus Disease 2019 Patients
4.2. Laboratory Results
The values of cardiac troponin I (cTnI), NT‐proBNP, and LDH were studied in all patients of mild, severe, and critical groups. The values of these indicators and their relationships with the clinical classification of the disease were analyzed and compared.
In severe and critical cases, the positive levels of LDH, cTnI, and NT-proBNP were higher than those in mild cases, and the differences between the groups were statistically significant (P value < 0.05). Cardiac troponin I was elevated (over 0.03 ng/mL) in 11 (15.1%) patients during hospitalization. The peak cardiac troponin I level (0.11 ng/mL [IQR: 0.33–0.20]), peak NT-pro BNP level (5840.35 pg/mL [IQR: 1609.39 – 10071.32]), and peak LDH level (578.65 UI/l[IQR: 313.40 – 843.90]) were significantly higher in the critical group (Table 3).
| Total, (N = 73) | Mild Group, (N = 39) | Severe Group, (N = 17) | Critical Group, (N = 17) | P Value | |
|---|---|---|---|---|---|
| Troponin (ng/ml) | 0.0320 (0.0105 - 0.0534) | 0.005 (0.002 - 0.006) | 0.005 (0.003 - 0.203) | 0.118 (0.033 - 0.203) | < .001 |
| < 0.03 | 62 (84.9 %) | 37 (50.7 %) | 17 (23.3 %) | 8 (11.0 %) | < 0.001 |
| ≥ 0.03 | 11 (15.1 %) | 2 (2.7 %) | 0 (0.0 %) | 9 (12.3 %) | |
| NT-pro BNP (pg/mL) | 1432.14 (361.99 - 2502.29) | 80.74 (46.66 - 114.83) | 124.18 (72.27 -176.08) | 5840.35 (1609.39 - 10071.32) | < 0.001 |
| < 125 | 44 (60.3 %) | 32 (43.8 %) | 9 (12.3 %) | 3 (4.1 %) | < 0.001 |
| ≥ 125 | 29 (39.7 %) | 7 (9.6 %) | 8 (11.0) | 14 (19.2 %) | |
| LDH (UI/L) | 326.89 (259.27 - 394.51) | 239.21 (215.52 - 262.89) | 276.29 (224.03 - 328.56) | 578.65 (313.40 - 843.90) | < 0.001 |
| < 250 | 38 (52.1 %) | 28 (38.4 %) | 9 (12.3 %) | 1 (1.4 %) | < 0.001 |
| ≥ 250 | 35 (47.9 %) | 11 (15.1 %) | 8 (11.0 %) | 16 (21.9 %) |
Coronavirus Disease 2019 Patients’ Levels of Cardiac Troponin, LDH, and NT-proBNP
4.3. Clinical Outcomes
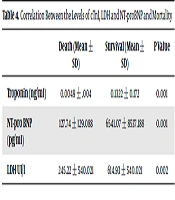
Until April 24, 2020, the overall Case Fatality Rate (CFR) was 20.54% (15 deaths among 73 cases). The CFR was 47.05% (8 deaths among 17 cases) in the critical group, which was not much higher than the CFR of 41.17% (seven deaths among 17 cases) in the severe group. Then, the levels of cTnI, LDH, and NT‐proBNP were compared between dead (N = 15) and surviving (N = 58) patients with COVID‐19. The cardiac injury biomarkers were significantly higher in the deceased group than in the survivor group (P value < 0.005) (Table 4).
| Death (Mean ± SD) | Survival (Mean ± SD) | P Value | |
|---|---|---|---|
| Troponin (ng/ml) | 0.0049 ± .004 | 0.1322 ± 0.172 | 0.001 |
| NT-pro BNP (pg/ml) | 127.74 ± 129.088 | 6541.07 ± 8537.188 | 0.001 |
| LDH UI/l | 245.22 ± 540.021 | 614.93 ± 540.021 | 0.002 |
Correlation Between the Levels of cTnI, LDH and NT-proBNP and Mortality
5. Discussion
Influenza infection is a common risk factor for chronic cardiovascular disease. Previous epidemics have been associated with heart injury. Acute myocardial infarction, acute myocarditis, and sudden heart failure have been described in SARS and MERS (8, 9). The novel Coronavirus Disease 2019 (COVID-19) has attracted great attention around the world. Previous studies suggest that severe COVID-19 may lead to acute heart damage (7, 10, 11). Huang et al. reported that 12% of patients with mild and severe COVID-19 had increased hypersensitivity to troponin I, suggesting acute myocardial injury (10).
In this study, the association of COVID-19 with heart injury was confirmed, and the correlation of the severity of SARS-CoV-2 infection and cardiac involvement was analyzed. We focused on the biomarkers of heart injury in mild, severe, and critical COVID-19 patients. We proved the elevation of cTnI, Pro BNP, and LDH in the critical group. In the critical group, 12.3% of the patients had a cTnI level above the reference level while it was 2.7% in the mild group. Moreover, the difference in the level and positivity rate of LDH and NT-proBNP was statistically significant among the three groups. This suggests that a rise in heart injury markers could be a potential indicator of critical disease and predict the severity of COVID-19. Also, the high values of cTnI, Pro BNP, and LDH in the group of dead patients compared to survivor patients indicated a higher fatality risk associated with the increased level of heart injury markers.
Retrospective cohorts have shown that the increased levels of cardiac injury markers at the onset of disease are associated with a more severe prognosis (12, 13). Guo et al. (3) could prove a direct relationship between troponin and the levels of highly sensitive C-reactive protein (CRP), an important inflammatory marker that strengthens the link between inflammation and myocardial damage. This fact should be raised as a warning because the risk of death from myocardial injury exceeds that of factors such as age, the presence of diabetes, and previous chronic lung and heart disease.
Despite the strong evidence, there is still no proof for the presence of the virus in the myocardium; however, we suggest the occurrence of direct and indirect heart injury attributed to the virus. Indirect damage can be caused by a cardiac overload due to systemic inflammation and hypoxemic respiratory failure whereas direct lesions would be caused by tissue infection leading to the death of cardiomyocytes (14). The finding of inflammatory infiltrates of mononuclear cells in autopsies in cardiac tissue is another argument suggesting direct cardiac injury by COVID-19 (15). In the study by Yi Han et al., the LDH elevation was positively associated with cTnI and BNP and showed that LDH could be identified as a strong predictor for the early identification of severe cases of COVID-19 (16). In another study by Zhou et al., the elevation of cTnI and LDH in critical cases was proven (17).
In a single-center study by Han et al. in Wuhan, China, the roles of cardiac troponin I and NT-pro BNP were studied. The authors assessed the results of 273 patients with COVID-19 and indicated that higher concentrations of these enzymes were associated with the severity of the disease and poor outcomes (18). Cardiac troponin I is a sensitive marker for myocardial injury (19), and NT-pro BNP is an optimal biomarker for heart failure (20). The mechanism of cardiac injury markers’ elevation in COVID-19 infection is not fully understood. The underlying pathophysiology suggests a heart-inflammatory response because a large series of severe cases of COVID-19 show a parallel elevation of inflammatory markers in the acute phase, such as C-reactive protein (CRP), procalcitonin, and ferritin (21). This can present clinically as fulminant myocarditis. Another mechanism involves the angiotensin-converting enzyme 2 (ACE2), which is a human cell receptor that binds strongly to the SARS-CoV-2 protein Spike. ACE2 is highly expressed in the heart (22, 23). SARS-CoV-2 can mediate myocardial inflammation and damage associated with the down-regulation of the myocardial ACE2 system, which may be responsible for the myocardial dysfunction and adverse cardiac outcomes in patients with SARS (24). However, a recent pathologic study reported rare mononuclear interstitial inflammatory infiltrations into heart tissue without substantial myocardial damage in a patient with COVID-19 (15), which may suggest that COVID-19 may not directly affect the heart.
Myocardial damage and heart failures presented in critical cases before death may be due to other reasons, such as severe hypoxia, which may be responsible for myocardial ischemia, management of mechanical ventilation, multiple organ failure, severe unbalance in water and electrolytes, or irreversible metabolic acidosis, which would cause serious systemic disorders in COVID-19 patients. All of these factors can influence the heart and cause secondary myocardial damage and heart failure (25).
Some limitations existed in this study. Some data, such as magnetic resonance imaging or echocardiography data and cytokine level measurements, were lacking to determine the characteristics of myocardial injury. Our study was also limited to patients whose data were complete, which decreased our sample size.
In conclusion, although it has not been confirmed that the presence of the novel coronavirus is linked to direct cardiac damage, the elevation of cardiac markers should be considered as a warning sign. We have shown that increased biomarkers of heart damage are associated with worsening outcomes in patients with COVID-19, and could be a powerful predictor for the early detection of severe COVID-19 cases. Serious monitoring of myocardial enzymes is of great importance to prevent complications and mortality in COVID-19 patients