1. Background
Hepatitis B virus (HBV) is a public health issue. More than two billion people show evidence of HBV infection globally. In 2015, hepatitis B resulted in an estimated 887,000 deaths, mostly from cirrhosis and hepatocellular carcinoma (1-3). Infection is spread from infected subjects by contact with body fluids, containing the virus. Percutaneous injuries are the main routes of HBV transmission (i.e., contaminated needles) (4, 5).
Healthcare workers (HCWs) are a population at higher risk for HBV infection (6, 7), due to their potential contact with blood or body fluids and possible needle stick injuries. Infected HCWs could also represent a risk for their patients (8). More than 300,000 HCWs are exposed to contaminated body fluids, and about 66,000 of them develop an infection annually (9-11). Previous studies showed how the risk for HCWs to develop clinical hepatitis, following injuries from HBV-containing blood needles, was 22% - 31%. Moreover, the risk for developing seroconversion for anti-HBc was 37% - 62% if the source of patient’s blood was positive for hepatitis B surface antigen (HBsAg) and HBeAg (12).
Consequently, European countries recommend HBV vaccination for HCWs protection (13).
In Italy, HBV vaccine has become mandatory at birth since 1991, and the Italian National Immunization prevention plan 2017 - 2019 (INIPP) strongly recommends vaccination of HCWs (12, 14). According to actual evidence, unprotected HCWs must receive three doses of HBV vaccine, while subjects exposed to potentially infected body fluids should receive a four doses schedule (0, 1, 2, 12 months). Current evidence shows that most vaccinated HCWs develop a protective level of anti-HBs antibodies, but seroconversion should be verified after the primary cycle of vaccination or after the administration of a booster dose to confirm the protection (14-17).
However, although data are not systematically available, vaccination coverage is estimated to be very low among HCWs. Particularly, a 2-year Italian seroepidemiological study reported that the vaccination rate against HBV was only 70.1% among HCWs (18). Previous studies have well documented that nurses are, among HCWs, the occupational group with the highest risk of percutaneous injury and HBV transmission. Literature reported the prevalence of anti-HBc antibodies (which is a marker of past infection) in 6.2% of HCWs vs. 1.8% of blood donors, indicating nurses’ job as a relevant risk factor (19). In a recent survey, the rate of anti-HBc positivity among Polish nurses was 16%, with the duration of employment being related to increased risk of being infected (20). In Italy, after the introduction, by the European Parliament, of directive 2010/32/EU on the protection from risks associated with exposure to biological agents, most healthcare facilities have implemented specific protection strategies, including the adoption of needle stick prevention devices (NPDs) to protect HCWs from biological accidents (21).
2. Objectives
In the present study, we aimed to evaluate the serological status of HCWs employed at the teaching hospital of Rome Tor Vergata and their risk of occupational injuries after the adoption of directive 2010/32/EU.
3. Methods
This retrospective observational study, approved by the ethical committee of the teaching hospital of Rome Tor Vergata, was carried on by the occupational medicine service. The following population was included in the study: All healthcare operators (physicians, nurses, auxiliary health personnel, medical students, dentistry, laboratory technicians, and obstetrics) exposed to biological risk due to their job duties. A total of 539 HCWs were enrolled in the study from 1 January 2018 to 31 December 2018. HCWs were enrolled in the study during the periodic occupational medicine examination that in Italy is performed annually by law (legislative decree 81/08).
During the annual occupational medicine evaluation for each subject, we collected the following data: Age, gender, nationality, job task, and area of employment. Moreover, all study subjects received, by means of venipuncture, a complete serological evaluation for HBV, including hepatitis B surface antibodies (anti-HBs IgG), antibodies to hepatitis core antigen (anti-HBc IgG), and HBsAg. Blood samples were collected and analyzed from the Department of Laboratory Medicine by means of electro chemiluminescence immunoassay (ECLIA). According to the literature, anti-HBs titer was considered protective when the value was ≥ 10 IU/L (immune). On the other hand, we considered the anti-HB < 10 IU/L not protective, and those subjects were classified as susceptible to HBV infection.
Subjects who had positive HBsAg were considered affected by chronic HBV hepatitis. Operators with negative results for HBsAg but positive results for HBc IgG were considered to have a past HBV infection. Data regarding needlestick injuries were collected by the prevention service team during the same year. For each event, the following data were collected by means of a standardized questionnaire: Name of the operator, occupational data (job task and area), type of event (injury with a hollow needle, point, or sharp instrument), site of the lesion, and source patient (when known).
We calculated the percentage of serologically protected subjects among enrolled HCWs in relation to main personal and occupational characteristics. Moreover, to evaluate the frequency and severity (potential or actual) of needlestick injuries, we calculated the incidence of occupational injuries occurring among the study population during the year 2018 in relation to operators’ serological status and the rate of seroconversion for HBsAg, following the occupational exposure to potentially contaminated body fluids. Analyses were performed using STATA® software (version number 11). The ANOVA test was used to compare means for continuous variables. The chi-square test and logistic regression analysis were used to investigate dichotomous factors related to the immunity of HCWs. The results were considered statistically significant at a P-value of < 0.01.
4. Results
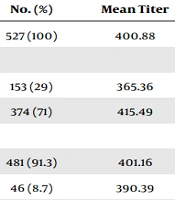
Data for 539 HCWs (mean age 40.7 years) were entered into the database. Table 1 shows the characteristics of the samples. HBsAg-positive and anti-HBc-negative subjects were 0.9% (five), and most of them were born in foreign countries (one in Africa, two in Albania, and one in Romania). None of those HCWs could document a written vaccination history. HBsAg-positive and anti-HBc-positive subjects were 1.3% (Seven) of the samples, of whom six were born in Italy. Among those subjects, three workers were born after the year 1980. Subjects were divided into two groups based on the anti-HBs titer: Group 1: 87.6% (462) of the samples had antibody levels higher than 10 IU/L, and Group 2: 12.4% (65) of the samples had antibody levels lower than 10 IU/L.
| Characteristics | No. (%) | Mean Titer | P-Value |
|---|---|---|---|
| Total number | 527 (100) | 400.88 | |
| Gender | |||
| Male | 153 (29) | 365.36 | < 0.01 |
| Female | 374 (71) | 415.49 | |
| Country of birth | |||
| Italy | 481 (91.3) | 401.16 | NS |
| Foreign | 46 (8.7) | 390.39 | |
| Age class (y) | |||
| < 40 | 227 (43.1) | 578.77 | < 0.01 |
| ≥ 40 | 300 (56.9) | 235.55 | |
| Job task | |||
| Nurse | 308 (58.4) | 504.55 | < 0.01 |
| Medical doctors | 212 (40.2) | 482.75 | |
| Other tasks | 7 (1.4) | 337.83 |
Demographic Characteristics and Mean Anti-HBs Titer of HBsAg and Anti-HBc-Negative HCWs (n = 527)
The risk of being serologically unprotected was higher in males and in subjects aged 40 years or over. Nurses seem more often protected than other tasks. Foreign birth was not significantly associated with unprotective anti-HBs levels. Logistic regression analysis is reported in Table 2. Regarding the risk of injuries, 16 accidents (five in males and eleven in females HCWs) involved in exposure to potentially contaminated biological fluids were reported during the year 2018. Ten out of 16 were punctures with contaminated needles, four out of 16 cases reported contamination of mucosal, and two out of 16 were represented by cuts with surgical devices. Thirteen out of 16 injuries involved nurses, whereas medical doctors and laboratory operators were rarely involved (2/16 and 1/16, respectively).
| Odds Ratio | Std. Err. | 95% CI | P > z | |
|---|---|---|---|---|
| Male gender | 2.14 | 0.66 | 1.22 - 3.75 | 0.007 |
| Age > 40 years | 3.47 | 1.14 | 1.81 - 6.63 | 0.000 |
| Nurse job | 0.42 | 0.13 | 0.22 - 0.78 | 0.006 |
| Foreign birth | 1.48 | 1.16 | 0.31 - 6.91 | 0.614 |
Factors Influencing Serological Susceptibility
Injuries occurred more frequently in emergency department (6/16), surgery (4/16), and medicine (4/16). One accident happened in a laboratory, and one injury in the radiological department. The hands were almost involved (13/16), while the conjunctiva was contaminated in 3 out of 16 cases. The overall risk of needlestick injury was 2.9% per year in the whole population, higher in nurses (4.2% per year) than in medical doctors (0.9%). At the time of injuries, 11 HCWs involved had HBsAb levels higher than 10 IU/L, and three were unprotected. None of these were positive for HBsAg and anti-HBc. There were no documented cases of seroconversion to HBV among the workers exposed.
5. Discussion
Protection against HBV infections remains a priority for all HCWs, regardless of their occupation. Occupational health services need to keep HCWs’ immunity to HBV through a prevention and control plan. The present study showed suboptimal levels of protection among HCWs, with a prevalence of protective anti-HBs titer under 88%, despite the efforts performed to ensure complete vaccination coverage and the vaccine offered among those subjects.
However, a percentage of those HCWs with a low anti-HBs titer should be considered protected. Previous studies have reported that even if anti-HBs titer declines over time below the protective level of 10 IU/L, immunological memory can persist. This protection is confirmed by the rapid increase in the anti-HBs level following the administration of a booster dose in subjects whose anti-HBs level had decreased below 10 IU/L (22-24). In our study, subjects aged 40 and over had a higher rate of unprotective antibody levels based on the decrease of anti-HBs titer over time after vaccination (24). The male gender was most commonly unprotected, and this finding was consistent with data from literature reporting a gender-specific difference in response to vaccination (25).
Nurses seem significantly more protected than other occupational duties, likely due to mandatory HBV vaccination for entry to nursing school. In addition, many doctors in our sample were in medical training and probably underwent an occupational medical examination for the first time and, therefore, might have already received a booster dose if needed. There are no studies in the literature on the cost-effectiveness of HBV immunization strategy among HCWs. Since the workplace pre-vaccination IgG screening and vaccination strategy proved high cost-effectiveness in a previous study, every effort must be made to identify unprotected individuals and offer them a complete vaccination schedule (26).
Directive 2010/32/EU legislates a framework agreement on the prevention of sharps injuries in hospitals and the healthcare sector. The EU Directive aims to achieve the safest possible working environment, to prevent workers’ injuries caused by all medical sharps, to protect operators at risk, to set up an integrated approach establishing policies in risk assessment, risk prevention, training, information, awareness raising, and monitoring and to put in place response and follow-up procedures. Where exposure cannot be eliminated, it should be prevented through the appropriate sharps disposal equipment, banning the practice of re-sheathing, implementing safe procedures for using and disposing of sharp medical instruments and contaminated waste, and eliminating the unnecessary use of sharps (21).
Risk of injury was relatively low in our population. Furthermore, the rate of past infections documented by IgG anti-HBc positivity was found to be the same as expected in the general population, according to recent findings that have shown a decrease in occupational risk of HBV in recent decades (27). Needle stick injuries in our population were relatively frequent, especially for nurses that had a greater than 4% annual risk of being exposed to potentially infected body fluids through percutaneous exposure. Our results did not compare the risk of injuries of HCWs before and after the adoption of the EU Directive, but indicated that, despite the adoption of that directive, the lifetime risk of biological injury and HBV infection for HCWs considered over a period of 30 years of employment, remained high. For this reason, every possible effort must be done to ensure that safety procedures are adopted, and all operators are immunized against HBV.
This research has some limitations: It is a retrospective study, and no data on the primary vaccination course was available. Moreover, the phenomenon of needlestick injuries could be underestimated since many operators may avoid the registration of minor injuries, particularly in case of contamination with body fluids from HBsAg-negative patients.
5.1. Conclusions
Our survey offers further insights into the risk of HBV infection among HCWs in Italy, following the introduction of mandatory vaccination at birth. The current study shows that the prevalence of HBV infection among HCW is similar to that of the general population. The high frequency of occupational injuries with possible exposure to HBV and the non-low percentage of HCW having a non-protective anti-HBs titer can result in a high risk of HBV transmission. Therefore, occupational health departments are responsible for increasing the levels of protection against HBV transmission in HCWs.