1. Background
The first human case of COVID-19 was officially reported in December 2019 in Wuhan (China) and caused the first pandemic of the new millennium. COVID-19 typically infects the upper respiratory and gastrointestinal tracts. The main presentations of COVID-19 include fever, myalgia, dry cough, dyspnea, diarrhea, and loss of smell and taste. Nevertheless, in severe cases, respiratory distress and end-organ damage are also common. Arbidol (Umifenovir) is an orally administered antiviral agent for treating influenza, SARS, and Lassa viruses licensed in Russia and China, which possesses immune-modulatory effects and a unique mechanism of action by targeting the S protein/ACE2 interaction and inhibiting the fusion of the viral envelope with the cell membrane (1, 2). Coronavirus enters the cell by ACE2 epithelial receptors, which causes activation of clathrin-mediated endocytosis that, in turn, changes the fluidity of membrane phospholipids and, subsequently, inhibits the virus entry into the host cell (1, 2).
2. Objectives
Since no definite treatment is developed for COVID-19 yet, the current study aimed to compare Arbidol for treating COVID-19 patients with mild to moderate and severe symptoms.
3. Methods
In this single-center, retrospective study, 47 patients (18 females and 29 males) with mild to moderate symptoms suffering from COVID-19 who were admitted to Labafinejad Hospital in Tehran, Iran, from March to April 2020 were investigated. Patients were separated into two groups of Hydroxicholoroquine and Kaletra as control (27 subjects; 14 females and 13 males) (1a) and intervention. Controls who had moderate symptoms received Hydroxicholoroquine and Kaletra (1a). The experiment group, who were 20 COVID-19 patients (16 males and 4 females) with mild to moderate symptoms were received Hydroxicholoroquine, Kaletra, and Arbidol (1b). Also, two groups comprised of 17 patients (13 males and 4 females) with severe symptoms of COVID-19 infection who received Hydroxicholoroquine, Kaletra, and Ribavirin as the control group (2a) and 17 patients (13 males and 4 females) with severe symptoms who received Hydroxicholoroquine, Kaletra, Ribavirin, and Arbidol (2b) were compared.
3.1. Data Collection
Information on age, sex, chronic medical illnesses (DM, HTN, …), presented symptoms (e.g. dyspnea, myalgia, cough, gastrointestinal upset), vital signs (e.g. temperature, pulse rate, respiratory rate, O2 saturation at the time of admission and on the fifth day of admission), duration of hospitalization, mortality rate, the ratio of neutrophils to lymphocytes, and the ratio of platelets to lymphocytes at the time of admission and on the fifth day of admission were collected.
The inclusion criteria were as follows: those aged 18 years and older with a probable or definitive diagnosis of COVID-19 who were candidates for hospitalization and receiving antiviral regimens, and having at least one of the COVID-19 symptoms (i.e. fever, chills, cough, myalgia, and dyspnea) with a positive RT-PCR test for SARS-COV-2 in a nasopharyngeal swab specimen or chest lung CT scan findings compatible with COVID-19 patterns.
To separate COVID-19 patients based on the disease severity (mild to moderate) the following criteria were used:
1) Critical (severe) COVID-19 patients met any of the following: respiratory rate more than 30 /min or O2 saturation less than 93% or a PaO2/FiO2 ratio lower than 300 mmHg or lung parenchymal involvement greater than 50%;
2) Mild to Moderate COVID-19 patients were patients with compatible clinical symptoms who did not meet the criteria for severe illness.
The Exclusion criteria were patients or his/her fellows' dissatisfaction to enter or continue the study, having a history or any sign of hypersensitivity to umifenovir (Arbidol), and pregnancy or lactation.
This study has been approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences in Tehran, Iran (IR.SBMU.RETECH.REC.1399.105).
4. Results
In the control group, there were 27 patients (14 (51.9%) females and 13 (48.1%) males) with mild to moderate symptoms who received Hydroxicholoroquine and Kaletra. The experiment group comprised of 20 patients (4 (20%) females and 16 (80%) males) who received Arbidol and Hydroxicholoroquine and Kaletra (group 1b). The mean age of participants in groups 1a and 1b was 61.5 and 60 years, respectively. All participants were at least 18 years of age.
There was no significant difference concerning the age and sex between the two groups (P values for age and sex were 0.812 and 0.36, respectively). 12 patients (44.4%) in the control group (1a) and 7 patients (35%) in the Arbidol treatment group (1b) were diabetics. 13 patients (48.1%, out of 27) in the control group and 6 patients (30%, out of 20) in the Arbidol group had Hypertension (Table 1).
| Group 1a | Group 1b | P Value | |
|---|---|---|---|
| Age, y | 61.5 | 60 | 0.812 |
| Gender (M/F) | 13/14 | 16/4 | 0.036 |
| Diabetic, % | 44.4 | 35 | 0.561 |
| HTN, % | 48.1 | 30 | 0.244 |
Twenty-two patients (81.5%, out of 27) in 1a and 10 patients (50%, out of 20) in 1b groups had positive nasopharyngeal swab tests, and the spiral chest CT scans of all patients were compatible with COVID 19 patterns.
The hospitalization duration for groups 1a and 1b was 9.3 and 10.6 days, respectively (P value = 0.327) (Table 2).
| Group 1a | Group 1b | P Value | |
|---|---|---|---|
| Fever (T > 37.5°C), % | 37 | 70 | 0.039 |
| Myalgia, % | 40.7 | 90 | 0.001 |
| Cough, % | 66.7 | 60 | 0.761 |
| Dyspnea, % | 44.4 | 100 | 0.000 |
| GI symptoms, % | 33.3 | 20 | 0.348 |
| PCR positive | 22 (81.5) | 10 (50%) | 0.008 |
| Mortality | 3 (11) | 0 (0%) | 0.251 |
| Duration of hospitalization | 9.3 | 10.6 | 0.327 |
aValues are expressed as No. (%).
Mean O2 saturation on the fifth day of admission in 1a and 1b groups was 91.9 and 94%, respectively. Intra-comparison of the two groups revealed no significant difference concerning the O2 saturation on the fifth day of admission (P value = 0.04). The mean temperature on the fifth day of admission was 37.3 and 36.4°C in groups 1a and 1b, which was statistically significant (P value = 0.07). Mean respiratory rate on the fifth day of admission in groups 1a (the Arbidol treatment group) and 1b was 20 and 19, which was statistically significant (P value = 0.015). The mean pulse rate on the fifth day of admission in (1a) was 91, while in (1b), it was 88. There was no significant difference between the two groups in this regard (P value = 0.519).
The ratio of neutrophils to lymphocytes (N/L) in the complete blood count on the first day of admission and the fifth day after admission in the 1a group was 5.3 and 6.4, respectively (P value = 0.769). The ratio of N/L in complete blood cell count on the first day of admission and five days after admission in the 1b group was 5.9 and 2.3, respectively. Comparing the N/L ratio in the complete blood count on the fifth day after admission in the Arbidol treatment group (1b) revealed a significant difference (P value = 0.024). The ratio of Platelets to lymphocytes (PLT/L) in the complete blood cell count on the first and fifth days of admission in the 1a group was 165 and 237, respectively. Furthermore, the ratio of PLT/L in the complete blood cell count on the first day of admission in 1b was 154, while on the fifth day it was 167. Intra-comparison of the two groups concerning the PLT/L revealed no significant difference between the first and fifth day of admission (P value = 0.103). The mortality rate in the 1a group was 11% (3 out of 27 patients), while no death was reported in 1b (P value = 0.251) (Table 3).
| Group 1a (on Admission) | Group 1a (Day 5) | Group 1b (on Admission) | Group 1b (Day 5) | P value on Day 5 (1a, 1b) | |
|---|---|---|---|---|---|
| Mean O2 saturation | 88.7 | 91.9 | 87.2 | 94 | 0.04 |
| Temperature | 37.7 | 37.3 | 37.1 | 36.4 | 0.007 |
| Respiratory rate | 23.7 | 20.8 | 21.9 | 19 | 0.015 |
| Pulse rate | 94 | 91 | 91 | 88 | 0.519 |
| N/L ratio | 5.3 | 6.4 | 5.9 | 2.3 | 0.024 |
| Plt/L ratio | 165 | 237 | 154 | 167 | 0.103 |
In the other 2 groups (2a, 2b), 17 patients (13 males (76.5%) and 4 females (23.5%)) with severe COVID-19 symptoms that received Hydroxicholoroquine, Kaletra, and Ribavirine were considered as the control group (2a). The experiment group was comprised of 17 patients (13 males (76.5%) and 4 females (23.5%)) who received Hydroxicholoroquine, Kaletra, Ribavirine, and Arbidol (2b). All participants were aged at least 18 years.
The mean age of participants in groups 2a and 2b was 62.9 and 65.3 years, respectively. 11 patients (64.7%) in 2a and 5 patients (29.4%) in 2b were diabetics. 7 patients (41.2%, out of 17) in 2a and 6 patients (35.3%, out of 17) in 2b had Hypertension (Table 4).
| Group 2a | Group 2b | P value | |
|---|---|---|---|
| Age, y | 62.9 | 65.3 | 0.552 |
| Gender (M/F) | 13/4 | 13/4 | 1.000 |
| Diabetic, % | 64.7 | 29.4 | 0.084 |
| HTN, % | 41.2 | 35.3 | 1.000 |
Four patients (23.5%, out of 17) in 2a and 10 patients (58.8%, out of 17) in the Arbidol 2b had positive nasopharyngeal swab tests and spiral chest CT scans of all patients were compatible with the COVID 19 patterns.
The duration of hospitalization in the 2a and 2b groups was 9.5 and 11 days, respectively (P value = 0.150) (Table 5).
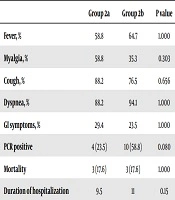
| Group 2a | Group 2b | P value | |
|---|---|---|---|
| Fever, % | 58.8 | 64.7 | 1.000 |
| Myalgia, % | 58.8 | 35.3 | 0.303 |
| Cough, % | 88.2 | 76.5 | 0.656 |
| Dyspnea, % | 88.2 | 94.1 | 1.000 |
| GI symptoms, % | 29.4 | 23.5 | 1.000 |
| PCR positive | 4 (23.5) | 10 (58.8) | 0.080 |
| Mortality | 3 (17.6) | 3 (17.6) | 1.000 |
| Duration of hospitalization | 9.5 | 11 | 0.15 |
aValues are expressed as No. (%).
Mean O2 saturation on the fifth day of admission in 2a and 2b was 88.3 and 88.1%, respectively. The intra-comparison of the two groups revealed that participants in the 2b group were not significantly different concerning the O2 saturation on the fifth day of admission (P value = 0.856) (Table 6). The results of the mean respiratory rate, pulse rate, and temperature on the fifth day post-admission are shown in Table 6.
| Group 2a (on Admission) | Group 2a (Day 5) | Group 2b (on Admission) | Group 2b (Day 5) | P value on Day 5 (2a, 2b) | |
|---|---|---|---|---|---|
| Mean O2 saturation | 87 | 88.3 | 86.5 | 88.1 | 0.856 |
| Temperature | 38.1 | 37.1 | 38.5 | 37.4 | 0.148 |
| Respiratory rate | 26.7 | 23.1 | 26.3 | 23.5 | 0.703 |
| Pulse rate | 95.7 | 91.4 | 93.8 | 90 | 0.199 |
| N/L ratio | 4.7506 | 4.6000 | 4.7381 | 4.6281 | 0.971 |
| Plt/L ratio | 180 | 173 | 183 | 163 | 0.719 |
The ratio of N/L in the complete blood cell count on the first day of admission in the 2a was 4.75 and on the fifth day of admission, this ratio was slightly decreased to 4.6. Also, the ratio of N/L in complete blood cell count on the first day of admission in the Arbidol group (2b) was 4.73 (P value = 0.986), whereas, on the fifth day of admission, it was slightly declined to 4.62 (P value = 0.971). The comparison of the N/L ratio on the fifth day of admission between the two groups revealed no significant difference (P value = 0.971) (Table 6).
The ratio of PLT/L in the complete blood count on the first and fifth days of admission in the 2a group was 180 and 173, respectively (P value = 0.909). Also, the ratio of PLT/L in the complete blood cell count on the first day of admission in the Arbidol group 2b was 183, while it was declined to 163 on the fifth day of admission.
Comparing the PLT/L ratio in complete blood cell count on the fifth day of admission in groups 2a and 2b revealed no significant difference (P value = 0.719) (Table 6). In group 2a that comprised of 17 patients with severe COVID-19, the mortality rate was 17.6% (3 patients), while in the Arbidol group (2b) it was almost similar (3 or 17.6%) (P value = 1.000).
5. Discussion
In this study, we evaluated the effectiveness of combined therapy with Arbidol in patients with mild to moderate symptoms of COVID-19 infection and also compared the impact of Arbidol in patients suffering from severe symptoms. Comparing the two groups (1a, 1b) that comprised of patients with mild to moderate symptoms, revealed that those who received Arbidol were significantly different concerning the O2 saturation on the fifth day of admission (P value = 0.04).
Also, in a retrospective cohort study conducted in China at the University of Zhejiang, Kaijin Xu et al. concluded that after administering Arbidol, patients’ need for high flow nasal catheter (HFNC) oxygen therapy was decreased compared to the control group (P value = 0.002). This indicates that Arbidol could accelerate viral clearance, improve radiological changes, and reduce the demand for oxygen therapy in hospitalized patients. It is noteworthy that these effects were particularly more prominent in patients with mild illness upon admission (3). In the present study, the Arbidol group was significantly different concerning the temperature on the fifth day of admission (P value = 0.07).
Chen et al. (4), in a study on the clinical effects of Arbidol combined with adjuvant therapy in China, concluded that the clinical symptoms of patients infected with COVID-19 were relieved faster and the duration of hospitalization was considerably reduced in the Arbidol group, compared to the controls (P < 0.05). In another study, Zhu et al. (5) in china reported no difference in the duration of fever between the two groups that were received arbidol and Kaletra (P = 0.61). 14 days after admission, viral shedding was significantly reduced in the Arbidol group. Also, the Arbidol treatment group was significantly different (P value = 0.015) concerning the respiratory rate on the fifth day of admission, but there was no significant difference between the two groups regarding the pulse rate on the fifth day of admission (P value = 0.519).
Regarding the N/L ratio in the complete blood cell count on the fifth day of admission, the two groups were significantly different (P value = 0.024), so that those in the Arbidol group had a better health status. The comparison of the PLT/L ratio in the complete blood cell count on the fifth day of admission revealed no significant difference between the 1a and 1b groups (P value = 0.103). In another study, Li et al. (6) investigated the safety and efficacy of Kaletra and arbidol on mild to moderate COVID-19 patients and reported no difference between the two groups regarding the improvement of clinical and radiological findings on the seventh day after initiation of treatment. In another study in China, Huang et al. (7) reported that Chloroquine and Arbidol could decrease the viral shedding interval and duration of hospitalization.
In the present study, no significant difference was observed regarding the duration of hospitalization between groups 1a and 1b (P value = 0.327). Furthermore, no significant difference was found between the two groups regarding the mortality rate (P value = 0.123). In a comparison between the groups of severely affected patients (2a, 2b), there was no significant difference in O2 saturation on the fifth day of admission (P value = 0.856). Besides, these groups displayed no significant difference regarding the temperature recorded on the fifth day of admission (P value = 0.148). Moreover, comparing the respiratory rates and pulse rates on the fifth day of admission in groups 2a and 2b revealed no significant difference (P values were 0.703 and 0.199, respectively).
In clinical lab tests, no significant difference was found concerning the N/L (P value = 0.971) and PLT/L ratios (P value = 0.720) between the two groups (2a, 2b). The findings showed that in severe and critical patients that required intensive care, Arbidol was not effective and did not have a significant impact on clinical outcomes.
5.1. Conclusions
This study showed that in patients with mild to moderate symptoms of COVID-19, treatment with Arbidol could decrease the duration of fever and improved the O2 saturation and the respiratory rate on the fifth day of admission. The ratio of N/L on fifth day of admission was significantly different in mild to moderate patients who received Arbidol, but in patients with severe symptoms of COVID-19, Arbidol was not effective in improving the O2 saturation and respiratory rate, or decreasing the temperature. Also, there was no significant difference concerning the N/L and the Plt/L ratios in severely infected patients.