1. Background
COVID-19 is a disease caused by SARS-COV-2, which was rapidly spread around the world after the outbreak in late 2019 in Hubei Province, China, and made pandemic conditions (1, 2). Currently, it seems that the individuals with underlying cardiovascular diseases, hypertension, underlying kidney diseases, and diabetes have more critical conditions compared to those with COVID-19 (3, 4). It is still unclear whether these underlying diseases themselves or the medicines that the patients take will cause an increased risk of COVID-19, but there are hypotheses on the effect of common medicines in these comorbidities (5).
Non-steroidal anti-inflammatory medicines, such as Ibuprofen, are now widely and used over the counter as antipyretics and analgesics (6). Moreover, NSAID consumptions due to their anti-inflammatory properties on the enzymes cyclo-oxygenases, which can alleviate the immune system of the patients with COVID-19, may have a negative impact on the severity of the disease (7). Accordingly, these hypotheses are valid for some NSAIDs like Ibuprofen, which have the ability to increase the expression of the enzyme ACE2 (5).
Fever is one of the most common complaints of the patients at the early stages of COVID-19 (8). In this regard, although NSAID consumptions can help in lowering the body temperature of these patients, its effect on the immune system and the reduction in cytokine storms can lead to worsening the COVID-19 symptoms (9). Therefore, despite sufficient evidence on the effect of NSAIDs on COVID-19 and based on the existing FDA and EMA hypotheses, it was recommended not to use NSAIDs unnecessarily (6). However, although several studies have been conducted on the use of NSAIDs for viral or bacterial pneumonia, no specific study has measured the effect of NSAIDs on COVID-19 yet (10-12).
2. Objectives
The purpose of this study was to investigate the historical effect of Ibuprofen on the severity of COVID-19 and mortality caused by this disease.
3. Methods
This study was conducted on 158 patients with COVID-19 who had consumed Ibuprofen for at least a week in the past three months. Accordingly, these patients were selected among the patients admitted to COVID-19 department of Shahid Mohammadi Hospital, Bandar Abbas, Iran, whose PCR tests’ results were positive. In addition, the patients with a history of renal artery stenosis, acute renal failure, hypercalcemia, and taking contrast during the past three months were excluded from the study. In this study, the patients were divided into three groups in terms of the severity of the disease as follows (13):
1) Mild: The patients who were hospitalized for less than 48 hours and had no hypoxia and O2 saturation ≥ 93%.
2) Moderate: The patients who were hospitalized longer than or equal to 48 hours, but required no hospitalization in ICU or were not expired.
3) Severe: The patients who need to be admitted to ICU or were expired.
ICU admission indications included O2 saturation < 93% at room temperature, severe respiratory distress, and respiratory rate > 30 beats/min. afterward, the relationship among the severity of the disease and the history of Ibuprofen consumption, diabetes, hypertension, history of cardiac problems, blood pressure, and GFR was investigated. Also, the relationship among the history of Ibuprofen consumption, GFR ≤ 60 mL/min, hypertension, history of cardiovascular problems, diabetes, LDH ≥ 500 U/L, lymphocyte count ≤ 1500, and mortality was investigated.
After the data collection, the obtained data were analysed by SPSS22 software. For data analysis, ANOVA test was used for the mean of quantitative variables and to obtain the adjusted OR and the factors affecting the severity of the disease, binary logistic regression command was used.
4. Results
In this study, 158 patients with COVID-19 were investigated in which were confirmed by PCR. The mean age of the patients was 52 ± 17 y/o, 74% of them were under 65 y/o. Notably, 54% of the patients were men and 46% were women.18% of the patients had a history of IHD, 35% had a history of HTN, 31% had a history of smoking, 19% of the patients had diabetes and 20% of them had a history of GFR less than 60 mL/min.
In this study, based on the duration of hospitalization and the need for ICU admission, the patients were divided into three categories as follows:
1) Mild: Hospitalization less than 48 hours (18 patients, 11%)
2) Medium: Hospitalization longer than 48 hours and no need for ICU admission (122 patients, 68%)
3) Severe: The patients admitted to ICU or expired (18 patients, 11%).
The mean age of the patients was 47 ± 18 years old in the mild group, 52 ± 17 years old in the moderate group, and 60 ± 16 years old in the severe group; however, no statistically significant difference was observed among these groups (P = 0.07).
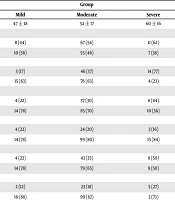
Regarding the relationship between the patients’ gender and disease severity, in the severe group, 61% of the patients were men and 39% of them were women. In the mild group, 44% of the patients were men and 56% of them were women. A statistical significant relationship was observed between the patients’ gender and disease severity (P < 0.001) (Table 1).
| Variable | Group | P Value | ||
|---|---|---|---|---|
| Mild | Moderate | Severe | ||
| Age | 47 ± 18 | 52 ± 17 | 60 ± 16 | 0.07 |
| Gender | 0.003 | |||
| Man | 8 (44) | 67 (54) | 11 (62) | |
| Woman | 10 (56) | 55 (46) | 7 (38) | |
| NSAIDs | < 0.001 | |||
| Yes | 3 (17) | 46 (37) | 14 (77) | |
| No | 15 (83) | 76 (63) | 4 (23) | |
| Smoking | 0.001 | |||
| Yes | 4 (22) | 37 (30) | 8 (44) | |
| No | 14 (78) | 85 (70) | 10 (56) | |
| DM | 0.004 | |||
| Yes | 4 (22) | 24 (20) | 3 (16) | |
| No | 14 (78) | 99 (80) | 15 (84) | |
| HTN | 0.017 | |||
| Yes | 4 (22) | 43 (35) | 9 (50) | |
| No | 14 (78) | 79 (65) | 9 (50) | |
| IHD | < 0.001 | |||
| Yes | 2 (12) | 23 (18) | 5 (27) | |
| No | 16 (88) | 99 (82) | 3 (73) | |
| GFR | < 0.001 | |||
| ≤ 60 mL/min | 2 (11) | 16 (14) | 14 (77) | |
| > 60 mL/min | 16 (89) | 106 (86) | 4 (13) | |
aValues are expressed as mean ± SD or No. (%).
In this study, regarding the relationship between ibuprofen consumption history and disease severity, a significant relationship was observed between disease severity and ibuprofen consumption history (P = 0.001). In the severe group including 14 patients, 77% of the patients reported a history of Ibuprofen consumption. Moreover, in the moderate group, 37% of the patients reported a history of Ibuprofen consumption and in the mild group, 16% of the patients reported such a history (Table 1).
In terms of the relationship between the severity of the disease and blood pressure in this study, it was observed that (50%) of the patients in the severe group, (35%) in the moderate group and 22% of the patients in the mild group reported a history of hypertension (P = 0.017) (Table 1). Also, regarding the relationship between kidney disease and the disease severity, the study found that, 77% of the patients in the severe group, 14% of the patients in the moderate group, and 11% of the patients in the mild group had GFR ≤ 60 mL/min (P < 0.001) (Table 1).
The study has also found a significant relationship between the severity of the disease and the history of ischemic heart disease (P < 0.001). Moreover, 77% of the patients in the severe group, 18% of the patients in the moderate group, and 12% of the patients in the mild group reported a history of ischemic heart disease (Table 1).
In this study, using logistic regression analysis, we investigated the factors affecting the patient’s mortality (Table 2). Ibuprofen consumption history, GFR ≤ 60 mL/min, history of diabetes, LDH ≥ 500 U/L, and lymphocyte count ≤ 1500 were among the factors affecting mortality in this study.
| Variables | P Value | Adjusted OR |
|---|---|---|
| History of NSAID consumption | 0.001 | 2 |
| GFR ≤ 60, mL/min | 0.014 | 6 |
| IHD | 0.37 | |
| DM | 0.01 | 3 |
| HTN | 0.25 | |
| Smoking | 0.12 | |
| LDH ≥ 500, U/L | 0.04 | 2.009 |
| Lymphocyte count ≤ 1500 | 0.01 | 4 |
5. Discussion
This is the first cross-sectional study investigating the effect of Ibuprofen consumption, on the severity of COVID-19. Our results show a significant relationship between Ibuprofen consumptions history before infected by COVID-19 and COVID-19 severity as well as the patient’s mortality (P value < 0.001, adjusted odd ratio: 2, respectively). In this study, a significant relationship was also found among the severity of the disease and the history of smoking, history of cardiovascular diseases, and GFR. Moreover, a significant relationship was also found among GFR ≤ 60 mL/min, mortality, diabetes, LDH ≥ 500 U/L, and lymphocyte count ≤ 1500.
Our results confirmed the hypotheses of Fang et al. (5) on the negative impacts of NSAID compounds on COVID-19. Correspondingly, they stated that, Ibuprofen compounds can facilitate COVID-19 infection due to the increased expression of ACE2, to which SARS-CoV-2 virus binds to. In addition, NSAIDs have been shown to be important due to two factors as anti-inflammatory properties over cyclooxygenases that inhibit the innate immunity and secondly their widespread use as antipyretics and analgesics (7, 14, 15). Russell et al. (12) found no strong evidence to support or oppose the use of NSAIDs in COVID-19 by reviewing the existing literatures. Despite the lack of adequate evidence for COVID-19, a study performed by Bourgeois et al. considered NSAIDs as an increased risk of empyema in children with acute viral infection (10). Also, a study by Basille et al. (11) showed that, the consumption of NSAID by young and healthy individuals before their admission due to the community-acquired pneumonia was related to the increased treatment time and greater pleuropulmonary complication. Although the above-mentioned results may confirm our results on the NSAID consumptions, the studied pathophysiology of the diseases may be different from COVID-19, and their results may be affected by the low sample size. In addition, investigation of the effect of some residual confounding factors such as lifestyle, smoking, underlying diseases, respiratory diseases, and COVID-19 can be important.
We also observed a significant relationship between IHD and COVID-19 severity (P < 0.001). Accordingly, a study by Mehra et al. (16) on the effect of underlying cardiovascular diseases on the increased risk of mortality among the COVID-19 patients in hospital can confirm our results.
Our studies showed a significant relationship between DM and mortality (P = 0.01, adjusted OR = 3). Moreover, a meta-analysis by Kumar et al. (17) confirmed our results on the effect of DM on mortality rate. Other studies also agreed on the increasing effect of diabetes on the mortality rate of the COVID-19 patients (18, 19). The prevalence rate of diabetes in COVID-19 patients was also calculated to be 9.8% (17).
By investigating the patients’ GFRs in our study, it was shown that, it also had a significant relationship with disease severity and mortality rate (adjusted OR = 31, P < 0.001), which is consistent with the results of a study by Wang et al. (20) on lower GFR levels in non-survivor group. Due to the high prevalence of kidney diseases, the ability to progress to acute kidney injury and its relationship with in-hospital mortality in the COVID-19 patients mentioned in various previous studies (20, 21). we suggest special care for those COVID-19 patients with kidney diseases.
It was also observed that LDH ≥ 500 U/L was significantly related to increased mortality (P = 0.04, Adjusted OR = 2.009). In this regard, our results were consistent with the results of other studies on the increase in LDH levels during the worsening of COVID-19 (22-24). Also, Zhao et al. (22) has specifically compared LDH in two groups, as COVID-19 and non-COVID-19 pneumonia, and then introduced LDH as a marker for evaluating the patients with COVID-19.
Other results showed a significant reduction in lymphocyte count related to the increased mortality (P = 0.01, adjusted OR = 4). Correspondingly, other studies have had similar results (25, 26). In a study by Henry et al. (25) a reduction was observed in lymphocytes in severe and fatal COVID-19 compared to non-severe disease. Similar results were found on the reduction in lymphocytes and the increase in leukocytes in a study by Qin et al. (27). Accordingly, in this study, although the number of T lymphocytes in general followed this trend, an increase was also observed in the percentage of native T-helper cells. In this case, further studies are recommended to better understand the pathophysiology of this disease. This study also reviewed the history of smoking and hypertension, which reached no significant level due to the small statistical population.
Notably, one of the limitations of the study was the low sample size, which reduced the statistical ability to detect some of the relationships. In addition, the inaccuracy of the daily dose of NSAIDs by the patients prevented the analysis of the effect of the medicines dose.
In this study, we were not able to diagnose pathophysiology and the exact molecular process of NSAIDs on the severity of COVID-19, so in this case, we were satisfied with the proposed hypotheses.
At the time of writing this article, we found no direct study conducted on the effects of ibuprofen consumptions on COVID-19 severity and mortality. Although this was a small sample size study, it is considered as the first study in this field and our results can be generalized to larger populations and suggest health policies during COVID-19 epidemic.
5.1. Conclusions
This is the first cross-sectional study investigating the effect of ibuprofen on the severity of COVID-19. It showed a significant relationship between the history of ibuprofen consumption before COVID-19 infection on the severity of COVID-19 as well as mortality rate of the patients, so this result could suggest health policies during COVID-19 epidemic.