1. Background
Prostate cancer is the most frequent cancer diagnosed and the second leading cause of cancer-related death among males all over the world, with an estimated 1,276,106 new cases and 358,989 deaths annually. During the last decades, the burden of prostate cancer has extensively increased (1, 2). The precise etiology of prostate cancer has not yet been determined. However, several factors are supposed to contribute to its development, including genetic background, lifestyle, environmental exposures, inflammation, and infections (3, 4).
It has been suggested that inflammation caused by infectious agents has a significant role in the regulation of tumor microenvironment and is associated with an increased risk of prostate cancer (5). Sexually transmitted infections by oncogenic viruses such as Human Papillomaviruses (HPVs) can cause cellular transformation in prostate tissue (6). Human papillomaviruses belong to the Papillomaviridae family, a group of small, non-enveloped, double-stranded, circular DNA viruses. They comprise more than 120 types, classified into low-risk (non-oncogenic) HPV types (including types 6, 11, 42, 43, and 44) and high-risk types (oncogenic; types 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 70) (7). Among them, almost 40 types (for instance types 16, 18, 31, 33, 35, 39, 45, 52, 56, 58, and 68) are well-known risk factors for the occurrence of cancer (7).
The HPV infection can be a cause of prostatitis (8). Moreover, HPV oncogenes (E6 and E7) target several cellular factors associated with cell-cycle regulatory systems. The E6 and E7 oncoproteins inhibit the function of tumor suppressor proteins p53 and pRb, respectively (9). Clinical and epidemiological evidence demonstrates the role of HPV in the development of other cancers (10, 11). However, there are contradictory findings of the role of HPV infection in the development of prostate cancer, which may be related to ethnic variations and different genetic backgrounds (12-14).
2. Objectives
Accordingly, the current case-control study aimed to investigate the association of HPV infection with prostate cancer development among Iranian men. Moreover, the correlation of HPV with prostate-specific antigen (PSA) level and disease aggressiveness was assessed.
3. Methods
3.1. Study Design
This case-control study was performed on 70 archival formalin fixed-paraffin embedded (FFPE) prostatic tissue blocks obtained from men undergoing either transrectal ultrasound (TRUS)-guided biopsy of the prostate or radical prostatectomy at our institution. Among them, 35 histopathologically confirmed prostate cancer tissues and 35 benign prostate hyperplasia (BPH) samples were enrolled in the study as cases and controls, respectively. The differentiation level of the prostate tumor (Gleason Score, GS) was assessed by a pathologist using the Gleason scoring system (well-differentiated, GS < 7; moderately differentiated, GS = 7; poorly differentiated, GS > 7) (15, 16). This study was performed following the principles of the Helsinki Declaration and was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1397.1096).
3.2. HPV Typing
The obtained specimens were examined by the polymerase chain reaction (PCR) and in situ hybridization. Genomic DNA was extracted from tumor tissues and BPH samples (as cases and controls, respectively) using QIAamp DNA FFPE Tissue Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s recommendation. A PCR assay was conducted for the detection of HPV according to the amplification conditions and primers described in a previous study (17). Briefly, consensus primers of MY09/MY11, GP5+/6+ with PCR product sizes of 450 bp and 150 bp, respectively, were used for the highly conserved late region I (L1) of the HPV genome, which encodes a viral capsid protein (18). The β-globin gene amplification was performed as the internal control (PCR product size: 268 bp), as described previously (19). Subsequently, HPV-positive specimens were subjected to hybridization with the consensus radiolabelled oligonucleotide probes to detect high-risk HPV types (16/18) and low-risk types (6/11). The detailed procedure was described previously (19).
3.3. Statistical Analysis
Statistical analysis was performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Quantitative data were compared using t-test and qualitative data using either Fischer's exact test or the chi-square test. These tests were considered statistically significant at P ≤ 0.05.
4. Results
4.1. Clinicopathological and Demographic Characteristics
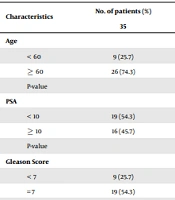
A total of 70 histopathologically confirmed prostate tissues comprising 35 BPH and 35 cancerous ones were selected as cases and controls, respectively. The age of prostate cancer cases ranged from 51 to 79 years (mean 64.9 ± 7.5 years), and that of BPH controls ranged from 50 to 79 years (mean 64.4 ± 6.2 years), with no significant difference between the two groups (P = 0.768). The PSA level ranged from 3 to 27 ng/mL (mean 9.93 ± 5.47 ng/mL) in cancer patients compared to 1 to 19 ng/mL (mean 8.86 ± 4.27 ng/mL) in BPH controls. The result of the independent t-test revealed that the mean PSA level did not differ significantly between cases and controls (P = 0.367). Among patients with prostate cancer, nine (25.7%), 19 (54.3%), and seven (20%) patients had GS < 7, = 7, and > 7, respectively.
4.2. HPV Prevalence
Statistical analysis revealed a significant association between the presence of HPV DNA and prostate cancer. The primary results of PCR for the L1 region revealed HPV infection in 34.3% and 8.6% of the cases and controls, respectively (P = 0.018). The HPV typing using in situ hybridization revealed a significant difference between the two groups of cases and controls in terms of infection with HPV types 16 and 18 (Table 1). In addition, none of the cases and controls were positive for HPV type 6. The results of the analysis of the relationship between HPV and clinicopathological and demographic characteristics in the case group are presented in Table 2. The findings showed no significant association of HPV infection with age and PSA level of the patients (Table 2). There was also no significant relationship between the age of patients and infection with different types of HPV (P > 0.05). In addition, no significant association was found between infection with HPV and GS (Table 2).
| Characteristics | No. of Patients (%) | HPV L1 (%) | HPV16 (%) | HPV18 (%) | HPV11 (%) |
|---|---|---|---|---|---|
| No. (%) | 35 | 12 (34.3) | 7 (20.0) | 4 (11.4) | 1 (2.9) |
| Age | |||||
| < 60 | 9 (25.7) | 5 (55.6) | 3 (33.3) | 2 (22.2) | 0 (0.0) |
| ≥ 60 | 26 (74.3) | 7 (26.9) | 4 (15.4) | 2 (7.7) | 1 (3.8) |
| P-value | 0. 220 | 0.306 | 0.204 | 1.000 | |
| PSA | |||||
| < 10 | 19 (54.3) | 6 (31.6) | 4 (21.1) | 2 (10.5) | 0 (0.0) |
| ≥ 10 | 16 (45.7) | 6 (37.5) | 3 (18.8) | 2 (12.5) | 1 (6.3) |
| P-value | 0.736 | 1.000 | 1.000 | 0.458 | |
| Gleason Score | |||||
| < 7 | 9 (25.7) | 2 (22.2) | 0 (0.0) | 1 (11.1) | 1 (11.1) |
| = 7 | 19 (54.3) | 9 (47.4) | 6 (31.6) | 3 (15.8) | 0 (0.0) |
| > 7 | 7 (20.0) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 0 (0.0) |
| P-value | 0.223 | 0.137 | 0.787 | 0.583 |
5. Discussion
It has been established that high-risk HPVs may have a potential role in the development of cancer (20). The association of oncogenic HPV types (such as types 16 and 18) with the development of epithelial cancers has been suggested previously (11, 20-22). However, the role of HPV infection in the development of prostate cancer is controversial (12, 23, 24). In the current study, the HPV infection was evaluated in prostate cancer cases, and BPH controls.
The results of the present study indicated a significant association between HPV infection and prostate cancer (P = 0.018). Our finding is consistent with those of other studies performed worldwide (13, 18, 25, 26). Thus, it could be concluded that HPV infection potentially affects prostate cancer development. The immortalization of prostate epithelial cells with HPV types 16 and 18 has been shown previously (27). Moreover, the contribution of HPV to prostate cancer may indicate that chronic recurrent inflammation is triggered by infection, and subsequently, the prostate gland is infected because of the close anatomical juxtaposition to the urinary sites (18).
On the contrary, two previous studies performed in Brazil yielded opposite results and reported no significant association between HPV infection and prostate cancer (14, 28). The data from a previous study in Iran also showed the presence of HPV DNA in 17.2% of prostate tumor samples and reported no significant role for HPV infection to play in prostatic disease (29). Moreover, in an additional study that used serological and molecular methods, no significant association was found between infection with HPV and prostate cancer (26). Contradictory results of different studies about the association of HPV with prostate cancer could be due to the effect of different factors such as ethnicity, geographical area, and lifestyle (25).
In the present study, the prevalence of HPV cancer cases was found to be 34.3%, which was lower than that reported in some previous studies in other regions (19, 30). The former studies in India, Argentina, and California have reported a detection rate of HPV DNA in prostate cancer between 41% and 65% (19, 25, 30). The prevalence of HPV-16 and 18 in prostate cancer cases varies in different studies and ranges from 2 to 65% (31-34). We found the highest rate of HPV infection in subtype 16 (20%), and none of the cases and controls were positive for HPV type 6.
In our study, further typing of HPV demonstrated a significant difference in infection with high-risk HPV (types 16 and 18) between cases and controls (P = 0.003 and 0.028, respectively). In addition, we found that the majority of the cancer cases that were positive for HPV infection harbored high-risk HPV types 16 and 18. This result may reveal the role of high-risk types of HPV in the development of prostate cancer. This result was also in line with data from previous studies (35). The involvement of HPV type 16 has been reported in over 50% of genital infections and a great number of oropharyngeal and anogenital cancers (36). Moreover, neither the cases nor the controls were positive for low-risk HPV type 6 in the present study.
According to our results, although younger patients (< 60) had a higher frequency of HPV infections, there was no significant association between infection and HPV and age (P > 0.05, Table 2). In addition, patients with GS = 7 showed higher rates of infection with HPV. However, we did not observe any association between HPV infection and GS; whereas, a former study indicated that HPV infection was more prevalent in the advanced stage of prostate cancer (25). Our results in terms of the lack of association between GS and HPV infection are in line with the results of Martinez-Fierro et al. They also found no correlation between HPV infection and tumor aggressiveness (37).
We found that the prevalence of HPV in the BPH group was 8.6%, and only one of them was infected with high-risk HPV type. However, a study by Glenn et al. indicated the presence of high-risk HPVs in benign prostatic tissues before the development of prostate tumors in the same patients. They suggested that HPV has an oncogenic role in the early stage of prostate tumorigenesis (38).
Like other studies, our study also has some limitations, including the small sample size. Moreover, other high-risk genotypes of HPV were not studied, which are proposed to be investigated in future studies.
5.1. Conclusions
To conclude, the present study signifies the potential role of HPV infection, especially with high-risk types, in prostatic carcinogenesis rather than tumor progression or aggressiveness. The asymptomatic HPV-infected males can be considered as reservoirs and carriers of the virus to their sexual partners. Therefore, given the role of HPV in cervical cancer, it can be a risk factor for both men and their spouses (39). Additional studies are required to establish the association of this sexually transmitted virus and the pathogenesis of prostate cancer. Therefore, preventive measures such as vaccination against HPV infection (especially high-risk types) or screening and treatment of infected individuals can be directed as a strategy for limiting prostate cancer.